Dec 6 2016
 Operando X-ray spectroscopy set-up. Credit: HIMS.
Operando X-ray spectroscopy set-up. Credit: HIMS.
One of the main factors in enhancing the performance of electric vehicles is the enhancement of batteries. In order to achieve this, the research priority area of Dr Moniek Tromp (from the University of Amsterdam), i.e. Sustainable Chemistry, involves performing advanced X-ray spectroscopy of battery electrodes whilst in operation. In the recent past, this technique has given new understanding of the process accountable for capacity fading of Li-ion batteries. The findings were recently presented by Tromp at the Dutch chemistry conference CHAINS 2016 in Veldhoven.
Tromp collaborated with the Technical Electrochemistry research team of Prof. Dr Hubert Gasteiger at the Technical University of Munich (Department of Chemistry) to perform the research. The findings of the research have been recently reported in the Journal of Materials Chemistry A—a high impact journal on materials for energy and sustainability.
Li-ion batteries are mostly used in applications such as laptops and mobile phones. They also seem to be the future of electromobility because they have the ability to considerably enhance the driving range of electric vehicles. However, this mandates an increase in their energy density by at least a factor of 2.5. In order to achieve this, an array of cathode materials with high energy density has been developed. Among these materials, materials based on manganese oxide, such as LiNiMnCoO (NMC), seem highly favorable.
However, it is highly unfortunate that the capacity of Li-ion batteries including the new cathode materials is observed to be severely reduced. Cobalt, nickel, and manganese ions from the cathode dissolve and diffuse into the anode, thus causing irreversible side reactions that affect the optimal battery performance. Specifically, the accumulation of manganese on the anode was found to have a ruinous effect.
Therefore, in order to prevent or reduce the undesirable side effects and thus to optimize battery performance, it is vital to have a detailed mechanistic understanding of the anode side reactions. For this, it is highly important to determine the oxidation state of manganese, which in turn determines its reactivity. Recently, this has been the center of interest of various research projects, however with conflicting outcomes.
The Amsterdam and Munich researchers now provide a major contribution to this discipline by carrying out, for the maiden time, X-ray spectroscopy under operating conditions. Their findings undeniably exhibit that the oxidation state of the migrated manganese at the anode is +2, which holds for cobalt and nickel as well.
This outcome is in contrast to many earlier observations in which various oxidation states were found to range from 0 to +4. The researchers regard that these differences may be caused because earlier outcomes were acquired by means of ex situ analysis in which electrodes are analyzed after recovery from a working battery cell. Such procedures of electrode harvesting and preparation can considerably influence the observed oxidation state.
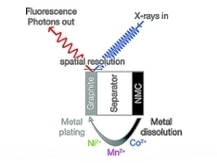
On the contrary, the new method of operando X-ray spectroscopy observes the oxidation states under battery operating conditions, thus providing a more realistic image of the electrode’s state and obviating the influence of electrode preparation processes such as washing and drying. The research was carried out at the SAMBA beamline of the Soleil synchrotron in Saint-Aubin, France, by employing dedicated X-ray absorption spectroscopy (XAS) electrochemical cells comprising graphite anodes and NMC cathodes, as developed by Tromp earlier.
The operando experiments enabled both the spatially resolved and time/voltage determination of oxidation state and metal concentration of the cobalt, nickel, and manganese deposits on the graphite electrode. It also indicated that NMC exhibited a strong increase in the metal dissolution rate when the upper cut off potential exceeded 4.6 V.
Thus, the research rendered a deep insight into the stability and deactivation of Li-ion batteries produced with NMC cathodes, demonstrating the significance of dedicated operando characterization for a comprehensive knowledge of the battery processes.
Further research in this field aims for highly stable battery systems, and for this, the focus is on the usage of varied transition metal compositions for the electrode as well as on the usage of electrolyte solvent additives to optimize the properties of transition metal dissolution.