The Geological storage of carbon is being considered as a promising method for minimizing the anthropogenic release of atmospheric greenhouse gases. This would involve sequestering of CO2 and other greenhouse-gases in underground reservoirs instead of releasing them into the atmosphere, which would add to global climate change.
There are a few challenges in performing CO2 sequestration, one of them is monitoring soil gases at the area of sequestrationto ensure continued integrity of the process.
Vibrational spectroscopic techniques, such as Raman and mid-infrared (IR) absorption spectroscopies provide a capable means to continuously monitor species such as water vapor, O2, CO2, CO, N2, NOx, SOx, and CH4 in soil gas for comparison with ambient levels, allowing evaluation of the integrity of sequestration areas in real time.
Differentiating between the variations in CO2 levels of that are indicitive of an issue with the sequestration site with those produced by natural phenomena, such as the respiration of plant roots, photosynthesis and the natural cycling of organic carbon in the soil, is considered the biggest challenge in gas sequestration monitoring.
Measuring the natural variation at the sequestration site is typically performed for a year or more in order to set up a baseline. Comparative analysis of CO2 within the borehole and in the local atmosphere is performed in subsequent monitoring. This article illustrates a practical means for comparison of CO2 concentrations at sequestration areas.
Experiment
The analysis of small homo- and hetero-nuclear soil gases can be performed using vibrational spectroscopy. The spectra provided using vibrational spectroscopy are clear and can be easily measured. Vibrational spectroscopy is also capable of acquiring spectra in real time with the help of other standard equipment and small sampling loops.
The experimental configuration used in this analysis consists of three main components: an external gas circulation module, a completed well (a finished well ready for injection) and several sensors (including an IR analyzer, a pressure sensor, a Raman spectrometer, and temperature and moisture sensors). Figure 1 schematically represents the gas-analysis system.
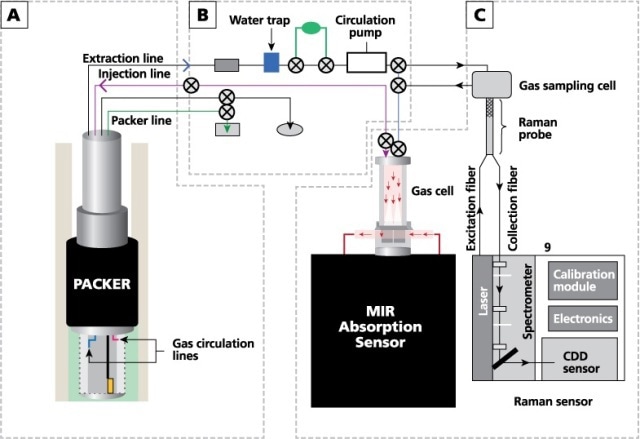
Figure 1. Schematic diagram of the gas-analysis system, showing (A) borehole and completion, (B) external module for gas circulation, and (C) IR and Raman analyzers.
Kaiser Optical Systems’ RamanRxn4™ gas-phase Raman analyzer equipped with an 532 nm laser was used to collect the Raman spectra. This setup offered a functional range of 175 to 4325 cm-1 for the Raman data.
An AirHead™ Raman probe head was mounted on the gas circuit which consisted of a custom gas cell coupled to the spectrometer using optical fibers. The custom gas cell is designed to use multi-reflection scattering amplification. This is achieved by focusing the laser with a sapphire window, reflecting the laser with mirrors to obtain the Raman backscatter which is then sent via the probe head to the spectrometer.
Results
A typical Raman spectrum of soil gas taken at the sequestration site is shown in Figure 2, with labels of key peaks for H2O vapor, N2, O2, and CO2. The Raman spectrum’s sharp, distinctive peaks allow different soil gases to be measured rapidly and accurately.
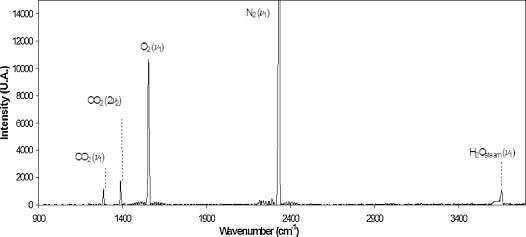
Figure 2. A typical Raman spectrum measured at the site in this study. Key peaks: H2O vapor (3657cm-1), N2 (2331cm-1), O2 (1555cm-1), and CO2 (Fermi dyad at 1388 and 1285cm-1).
Figure 3 displays the comparison of CO2 variation over a period of four days in January 2011 at the time of the injection operation. The comparison reveals that the Raman data is in good agreement with the data from spectral measurements.
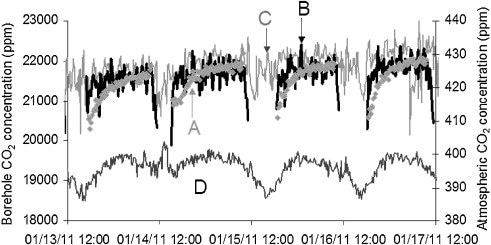
Figure 3. Diurnal variation of CO2 at the site and in the atmosphere over four days in January 2011. A, B are borehole CO2 concentrations calculated by IR, while C is the borehole CO2 concentration calculated from the Raman spectrum using the Fermi dyad at 1388 and 1285 cm-1. D is the atmospheric CO2 concentration measured by IR. (© 2013 Elsevier).
Conclusions
From the study results, it is evident that Raman spectroscopy is a feasible approach for monitoring soil gases at CO2 sequestration areas in real time. When the Raman spectrometer was coupled to a well completion, it could be used to effectively monitor concentrations of a number of significant soil gases, including CO2, at a sequestration site. In spite of of wet or dry conditions, continuous monitoring can be performed using Raman spectroscopy as it is not effected by liquid water. However, the mid-IR absorption spectroscopy cannot be used in wet conditions due to its broader liquid-water spectrum.
However, due to the high sensitivity of infrared in dry conditions, the Raman and the IR can be coupled together to be used in their own ideal conditions. Additionally when fiber coupling is used, it enables locating the Raman probe head within the completion, while still allowing the spectrometer base unit to be accessed in a surface module.
Installing a number of Raman probe heads at different locations at the sequestration site, such as near underground gas pipes, near injection wells, near abandoned wells for which integrity is a problem, in boreholes, and at natural sites such as fault zones and aquifers, allows for comparative analysis.

This information has been sourced, reviewed and adapted from materials provided by Kaiser Optical Systems, Inc..
For more information on this source, please visit Kaiser Optical Systems, Inc..