Sona is Andor’s latest high performance, vacuum cooled sCMOS camera platform, purpose built for fluorescence microscopy. Designed from the ground up to deliver unrivalled performance and versatility, the Sona platform debuts with the Sona 4.2B-11 and Sona 2.0B-11: the most sensitive back-illuminated sCMOS cameras commercially available.

Sona. Image Credit: Andor Technology.
The Most Sensitive Back-Illuminated sCMOS
The Sona 4.2B-11 and Sona 2.0B-11 back-illuminated sCMOS models both feature 95% Quantum Efficiency and Andor’s unique vacuum cooling feature, that allows temperatures of up to -45 °C, reducing noise to a minimum. Back-illuminated sensors are especially used for enhanced sensitivity, so it makes sense to choose the most sensitive adaption of this powerful technology.
- Reduce excitation power – preserve living specimens during observation
- Reduce fluorophore concentrations – obtain more accurate physiology
- Reduce exposure times – follow faster processes
The Largest Field of View
The Sona 4.2B-11 is Andor’s flagship model. It is a highly adaptable system which enables the capture of extremely large fields of cells or even whole embryos with superb clarity. Alongside this, the Sona 2.0B-11 maximizes the field of view available through C-mount microscope ports up to 22 mm. Pre-configured ROIs provide adaptability with 19 mm and 18 mm ports.
- Ideal for capturing large fields of cells, embryos and tissues
- High content imaging
- Developmental biology – capture whole embryos
- Tissue cultures – minimize stitching, maximize throughput
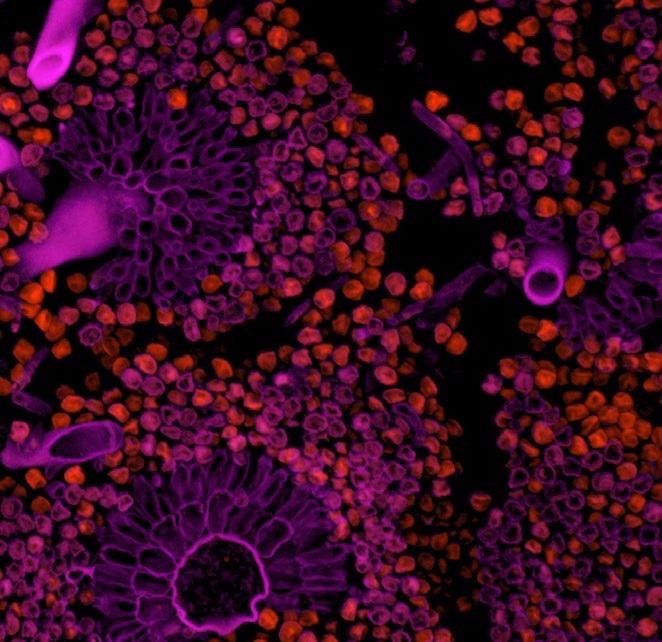
- Organoids – unravelling cell connectivities
- Gene editing – screen large cell cultures for successful phenotype expression
UltraVacTM - Why is Vacuum Technology Important?
Not only just reducing the noise floor to a minimum, Andor’s vacuum sensor enclosure delivers other benefits in the form of performance longevity:
Reason 1: Sensor Protection
Vacuum provides a protective barrier to back-illuminated silicon sensors that are otherwise susceptible to attack from moisture, hydrocarbons and other gas contaminants. Over time this can result in loss of performance, including QE decline.
Reason 2: No Re-Backfilling of Sensor Enclosure
UltraVacTM uses a hermetic vacuum seal, denying any entry by gas and moisture from the outside environment. This eliminates the issue of moisture condensation on the sensor and the hassle of then returning it to the factory for repair. Andor backs this up with a standard 5-year warranty on the Sona vacuum enclosure.
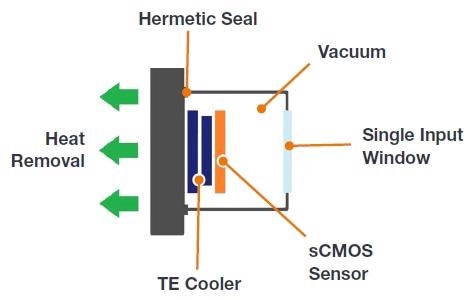
Andor’s UltraVacTM provides superior sensor protection and longevity.
Superior Field of View
The Sona 4.2B-11 flagship back-illuminated model is the embodiment of Andor’s unique approach to the technology that allows the user to usefully and uniquely access the entire 2048 x 2048 pixel array of the GSense 400 BSI sensor, giving an eye-catching 32 mm sensor diagonal.
When paired with the right objective matching, this allows the entire field of view available from the microscope to be harnessed. This is ideal for situations where large fields of cells, whole embryos or tissue samples are required to be captured with perfect clarity, for example in high content screening, organoid imaging and gene editing.
Quantitative Accuracy
Each of the Sona 4.2B-11 and Sona 2.0B-11 offer an Extended Dynamic Range (EDR) feature, supported by a 16-bit data range. Utilizing the novel ‘dual amplifier’ sensor architecture, the maximum pixel well depth and the lowest noise can be accessed simultaneously, ensuring quantification of incredibly weak and relatively bright signal regions in one snap. This functionality is convenient for imaging and quantifying many challenging samples, such as neurons.
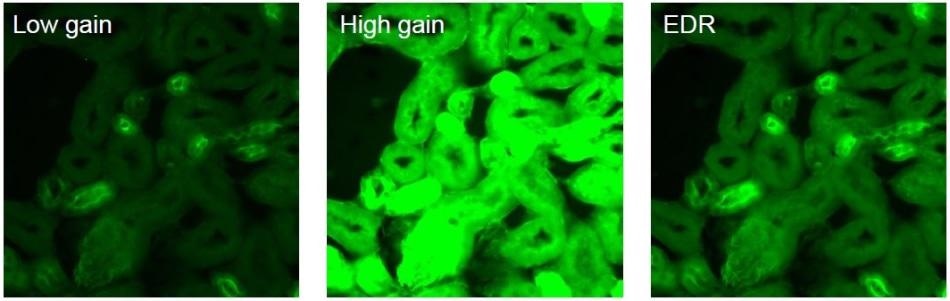
The same image compared under different modes. Low gain - captures brighter regions and accesses the max. pixel well depth. High gain – captures dim regions with minimum noise floor. Extended Dynamic Range - captures and quantifies both high and low signal regions, combining lowest noise and maximum pixel well depth.
Market-Leading Linearity
So as to achieve best-in-class quantification accuracy, Andor have implemented enhanced on-camera intelligence to deliver linearity of >99.7%.
In many cases, accurate quantitative information is crucial and required over just structural detail. This is the case for any measurement where intensity correlates to quantity or concentration will benefit from superior linearity.
For example:
- Physiological parameters such as calcium, pH, cAMP or PIP3 levels
- FRET analysis, such as for distance or co-localization measurements at the nanometer scale
- Gene expression analysis with fusion proteins
- Localization Super-Resolution Microscopy for better Gaussian fit
Rapid Frame Rates
Sona 4.2B-11 and Sona 2.0B-11 are enabled with fast frame rate capability, making them perfectly suited for following dynamic cell processes such as ion signaling, cell motility and blood flow, all without the image smear that can befall slower frame rates. Region of Interest (ROI) and 12-bit readout mode can be harnessed to bring about a further boost to frame rates.
Application Focus
Developmental Biology
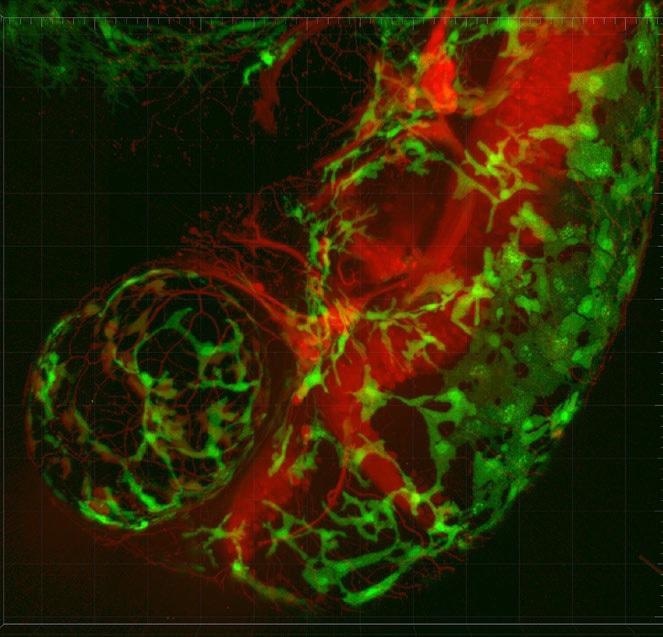
Imaging is a central technique in following the entire lifespan of organisms to track fates of developing cells, tissues and organs. By imaging whole-embryo and whole-body examples of well-established model organisms including the zebrafish and C. elegans, insight is given on various interconnected functional networks that then shed light on nerve impulse propagation in neural circuits or ventricular pacemakers in heart models. Sona 4.2B-11 allows these developmental specimens to be studied in the necessary large fields of view, for example using the Light Sheet Microscopy technique.
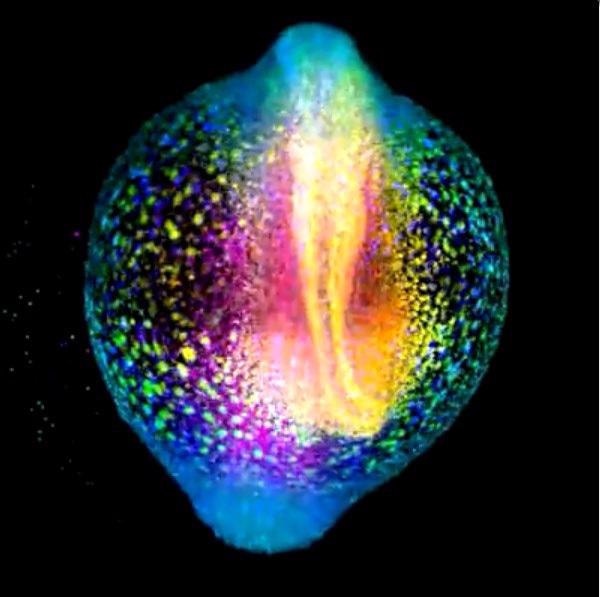
A developing zebrafish embryo imaged from 4 to 18 hours post fertilization where each cell nucleus is labelled with GFP. Cells are colorcoded for depth to visualize how dynamic cell reorganization gives rise to the body axis of zebrafish. Image courtesy of Gopi Shah, Max Planck Institute of Molecular Cell Biology and Genetics, Dresden.
Gene Editing
There has been a steady increase in recent years in the number of publications regarding the Crispr-Cas9 system, where this novel and versatile tool has been employed with great precision for DNA editing and the huge array of applications that benefit as a result of this.
As the back-illuminated deep cooled Sona sCMOS cameras offer best in class sensitivity, they are ideal for the imaging of Crispr-Cas9 constructs, perfect for fast and sensitive detection of light emitted by labelled DNA/RNA or related proteins involved in strand cleavage and modification of the existing genetic code. Screening of large cell cultures for successful gene edits is also made easier with the large field of view.
Plasma Membrane Dynamics
Analysis of phenomena associated with the plasma membrane is critical for many biological models involving cell adhesion, cell-to-cell communication and signal transduction, as well as cell fate differentiation.
There are many ways of imaging the plasma membrane, some of which use lipophilic or voltage sensitive dyes to directly label the membrane. By using the rapid frame rate, highly sensitive back-illuminated Sona cameras, this rapid remodeling of the plasma membrane can be captured, with the equipment being perfectly suited to the low light conditions inherent to TIRF Microscope.
Intracellular Trafficking
The ongoing traffic of molecules is vital to the continued operation of the cell’s finely tuned machinery, and without mechanisms in place it would immediately grind to a halt. Fast and sensitive imaging is necessary in order to study endosome cycling, Golgi vesicles pathways, axonal transport, hormone release or synaptic vesicle pool replenishment.
Andor sCMOS cameras have long been the go to detector for experiments involving imaging of cellular traffic. This continues with the new Sona 4.2B-11 and 2.0B-11 models, as their large FOV, resolution and speed are perfect for tracking intricate events and dependencies occurring within the cell’s transport and communications networks.

Bovine pulmonary artery endothelial cells (BPAEC) imaged with Andor Sona 4.2B-11 on a Nikon Ti2 inverted microscope with a x60, 1.4 NA objective lens. MitoTracker Red was used to stain the mitochondria in the live cells, with accumulation dependent upon membrane potential. Following fixation and permeabilization, F-actin was stained with Alexa Fluor 488 phalloidin, and the nuclei were counterstained with the bluefluorescent DNA stain DAPI.

This information has been sourced, reviewed and adapted from materials provided by Andor Technology Ltd.
For more information on this source, please visit Andor Technology Ltd.