Key Issues
- Compliance with EU REACH legislation
- Complementary information with other common methods of gas analysis
- Simple analysis of many gas-phase species including homonuclear diatomics
Introduction
REACH is a European Union regulation which is concerned with the Registration, Evaluation, Authorization, and Restriction of Chemicals.1
The regulation came into force in June 2007, aiming to ensure adequate protection of both the environment and human health from the inappropriate use of chemicals, while making the manufacturers and importers of chemicals responsible for the comprehension and management of any risks associated with their use.
REACH requires manufacturers or importers to register substances with the European Chemicals Agency (ECHA), and this process requires a thorough, comprehensive set of analytical data to verify the material’s identity.
Generally, the ECHA requires an absolute minimum of FTIR, UV-VIS and NMR or mass spectrometry to authenticate molecular structures, alongside LC or GC to evaluate impurities. In some instances, alternative or additional techniques such as X-ray fluorescence or X-ray diffraction may be necessary.
It is relatively easy to find suitable techniques for the characterization of solids and liquids in order to meet the requirements of ECHA, however, gases present a particular challenge. This is because many of the techniques previously mentioned are not ideal for the characterization of gases.
Mass spectrometry, GC and FTIR can provide very useful information, but NMR spectroscopy of gases is not common. Meanwhile, UV-VIS offers limited information unless the gases include potent chromophores.
One common concern is that ECHA may reject applications only supported by three analytical techniques, meaning that there is a genuine need for other methods suitable for characterizing gases.
Raman spectroscopy has a lot of potential in this respect, since it enables observation of homonuclear diatomics (which are infrared inactive) and provides comparable yet complementary information to FTIR spectroscopy - an approved tool for REACH registration.
Raman Measurement of Gases
Raman scattering from vapors or gases at atmospheric pressure tends to be weak due to the low density of molecules present in the sample. However, a novel probe design – the Kaiser Raman AirHead - facilitates the acquisition of high quality data from gases in relatively short timescales.
The examples below utilize an AirHead probe connected to a Kaiser Raman Rxn Systems™ analyzer fitted with a 100 mW, 532 nm diode-pumped Nd:YAG laser and a Peltier-cooled CCD detector. Figure 1 displays the sample-handling system used. This Raman analysis was undertaken in situ with the cell in the gas handling line.
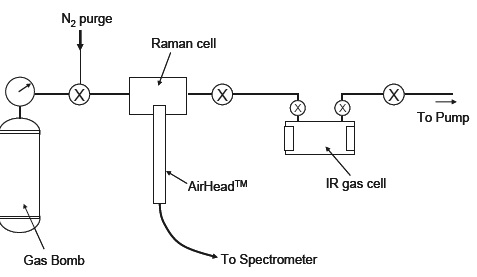
Figure 1. Schematic diagram of gas-handling system. Image Credit: Kaiser Optical Systems, Inc.
CH4 / H2 Mixture
Figure 2 displays the spectrum acquired from a sample of methane which contained ~1% hydrogen. The sharp, strong rovibrational band patterns close to 3000 and 1500 cm–1 are a highly characteristic fingerprint of methane gas.2 The single band close to 4159 cm–1 is the H2 stretching vibration.
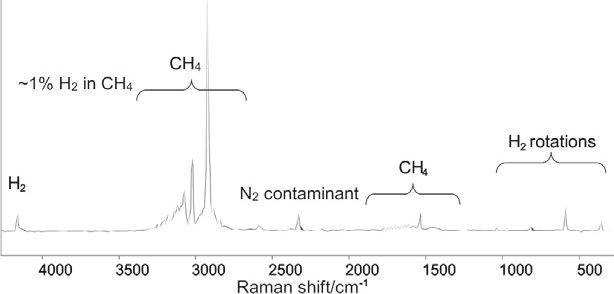
Figure 2. Raman spectrum of 1% H2 in CH4. The sharp bands below 1040 cm–1 are the pure rotational transitions of the H2 molecule. Image Credit: Kaiser Optical Systems, Inc.
This particular mode is inactive in the infrared spectrum because of the lack of change in the H2 dipole moment throughout the vibration. In this instance, FTIR spectroscopy (which is the standard ECHA-requested method for vibrational spectroscopy) would detect methane easily, but it would not provide an accurate picture of the material composition, as it is unable to detect the hydrogen component.
Alongside the H2 stretching band, a series of sharp bands can be seen between 1040 cm and 350 cm–1. These bands are pure rotational transitions of the H2 molecule3, with these transitions tending to occur at higher wavenumber than for other species, as H2 has a low moment of inertia. Because of the intrinsic strength and distinctive pattern of the band it is difficult to detect H2 at just 1% concentration in CH4.
A lack of gas-phase-capable technology suitable for the quantitative measurement of homonuclear diatomics forms a significant gap in current analytical technologies. This gap can be filled by using Raman spectroscopy
Isobutylene and Butadiene
Figure 3 displays Raman spectra from two samples of unsaturated C4 gases, acquired by averaging six scans of 30 second duration. The spectrum of the first sample (upper trace, blue) can be seen to be consistent with isobutylene.
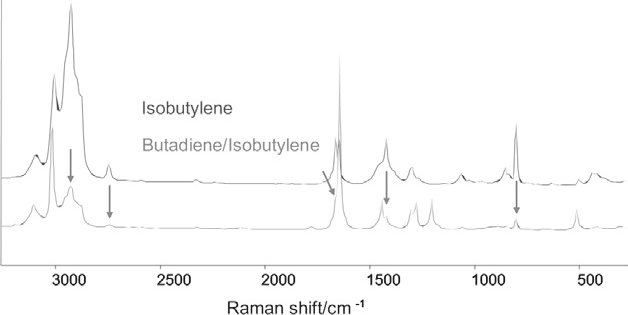
Figure 3. Raman spectra of C4 gases. The upper trace is consistent with isobutylene; the lower trace has strong bands due to butadiene and several weaker bands (arrowed) due to isobutylene. Image Credit: Kaiser Optical Systems, Inc.
Meanwhile, the lower trace confirms that the second sample is primarily butadiene,2 but a number of additional weak bands (highlighted with vertical arrows) verify the existence of isobutylene as a minor component.
Other Gases
Lastly, to demonstrate the usefulness of the AirHead probe in the analysis of simple gases, Figure 4 displays spectra of three more hydrocarbon samples. Each of these spectra was acquired by averaging six scans of 30 seconds duration.
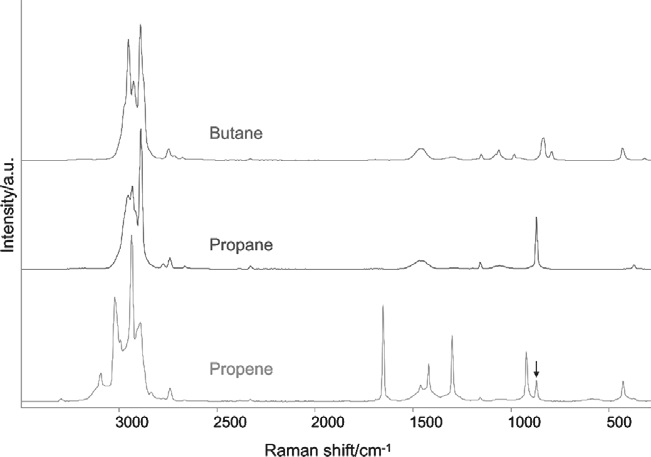
Figure 4. AirHead™ spectra of three common hydrocarbon gases. A weak band near 870 cm–1 suggests a minor propane impurity in the propene. Image Credit: Kaiser Optical Systems, Inc.
Here, the upper and middle traces exhibit consistency with n-butane and propane, while the sample in the lower trace is primarily propene, with a minor propane impurity.2
Conclusion
The examples above show that gases can rapidly yield high quality Raman spectra. Additionally, the Raman spectrum is a unique fingerprint for quantitatively analyzing mixtures and for verifying molecular structures.
Raman spectroscopy and IR are subject to different selection rules, meaning that the use of both techniques is advised in order to achieve comprehensive characterization of a sample. Relying solely on either one technique could result in a misleading impression of the sample’s composition.
As outlined above, Raman spectroscopy enables effective observation and quantitation of homonuclear diatomics, which are inaccessible to IR analysis. Overall, Raman spectroscopy is a valuable tool in supporting REACH registration of liquids and solids.
References
- www.hse.gov.uk/reach/about
- Schrader, B. Raman/Infrared Atlas of Organic Compounds, VCH: New York, 1989.
- Marowsky, G., et al. Applied Physics B, 39, 1986, 47–53

This information has been sourced, reviewed and adapted from materials provided by Kaiser Optical Systems, Inc..
For more information on this source, please visit Kaiser Optical Systems, Inc..