This article explores applications of the scTRACE Gold electrode, particularly after modifying the gold micro-wire with a thin layer of another metal.

Image Credit: Metrohm AG
Why Modify the Electrode Material?
Stripping voltammetry is a two-step measurement, with the first step requiring the analyte to be deposited on the working electrode. Anodic stripping voltammetry (ASV) involves the analyte being reduced before forming an alloy with the electrode material (Figure 1). Meanwhile, adsorptive stripping voltammetry (AdSV) involves the analyte forming a complex, which is then adsorbed to the working electrode.
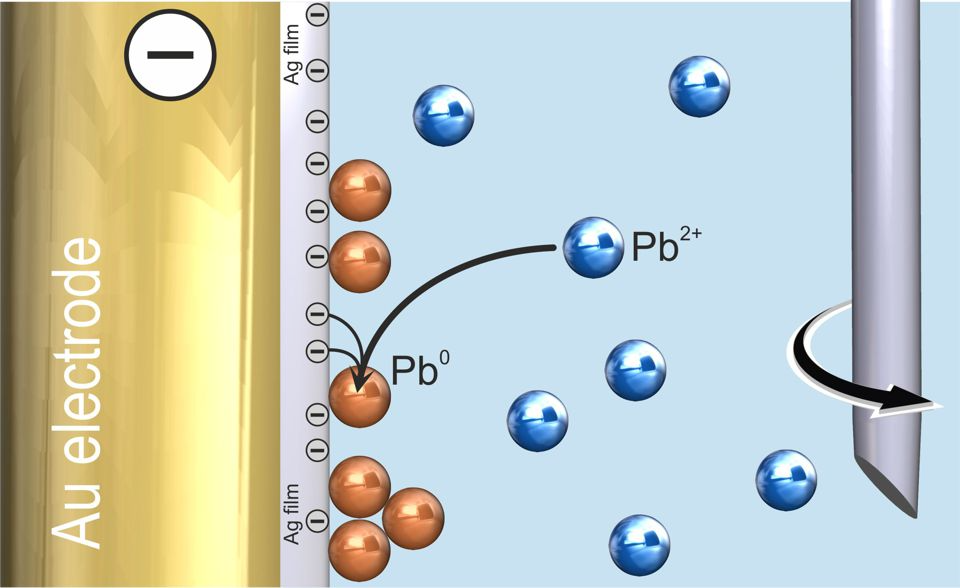
Figure 1. Anodic stripping voltammetry (ASV) with Ag film modified scTrace Gold electrode – deposition of lead (solution stirred). Image Credit: Metrohm AG
In the following stripping step, the deposit is returned into solution, providing an analytical signal which is proportional to the deposited amount of analyte. In the case of ASV, the electrochemical reaction is the re-oxidation of the analyte throughout an anodic scan (Figure 2). In the case of AdSV, the adsorbed metal complex is reduced during a cathodic scan.
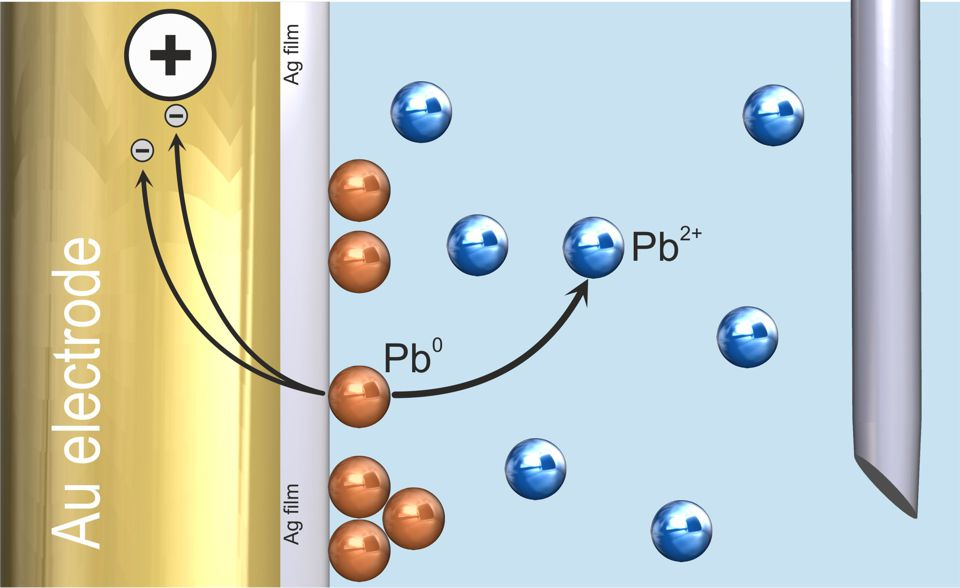
Figure 2. Anodic stripping voltammetry (ASV) with Ag film modified scTrace Gold electrode – stripping of lead (solution not stirred). Image Credit: Metrohm AG
In each step, stripping and deposition are subject to the principles of thermodynamics and kinetics. While there is no scope to explore the reasons why in this article, certain electrode materials are not suitable for determining some analytes. One means of solving this problem is to modify an existing working electrode with a different, more suitable material.
Applications
Lead in Drinking Water
Much of the lead present in ground and surface water is anthropogenic in origin, the result of leaching contaminated soils. However, lead in tap water tends to originate from household plumbing systems, with many countries manufacturing pipes from lead metal until the 1970s.

Image Credit: Metrohm AG
Lead is hardly soluble in water, but it does slowly dissolve in the presence of oxygen. The permissible limit for lead in tap water may be exceeded by a significant amount, and while lead pipes for municipal water transport are forbidden, some houses do still have old installations intact.
The World Health Organization recommends a limit value of 10 µg/L for lead in drinking water, while in the European Union, the upcoming limit could be as low as 5 µg/L.
The scTRACE Gold electrode can be modified with a silver film to enable the determination of lead. The film is plated ex-situ from a discrete plating solution and once plated, it is possible to use the film for multiple determinations. The film can be removed when it is depleted, and a fresh film added for further determinations.
A positive side effect of the silver film is that the scTRACE Gold electrode will last longer because aging processes will primarily affect the renewable silver film. A repeatability study revealed that determining 10 µg/L Pb using three different electrodes on four different days (10 determinations in total) resulted in the average recovery of Pb is 96% with a relative standard deviation of 5%.
The 884 Professional VA can measure lead concentrations in water down to 0.4 µg/L, facilitating a robust, straightforward determination that can comfortably accommodate the European Union’s future limit.

Image Credit: Metrohm AG
The 946 Portable VA Analyzer enables a limit of detection that is only marginally higher: 0.6 µg/L. Mobile use offers close monitoring possibilities for individual installations, with no need to preserve samples and transfer these to a central lab. Mobile use offers the additional benefit that concerned residents receive immediate results.

Image Credit: Metrohm AG
Nickel and Cobalt in Drinking Water
Like lead, nickel concentrations found in water sources can also be increased through human influence. Faucets and plumbing fixtures are often plated with a thin layer of nickel, protecting them against corrosion, even in installations where the finish is made of chromium.
Nickel is also part of many alloys, ranging from bronze and stainless steel to nickel brasses. The use of nickel pigmented dishes or nickel steel alloy cookware may also lead to increased nickel levels. The European Union recommends a maximum of 20 µg/L nickel in drinking water whereas the World Health Organization advises a limit of 70 µg/L.

Image Credit: Metrohm AG
An ex-situ plated bismuth film on the scTRACE Gold electrode is used as a working electrode or the voltammetric determination of cobalt and nickel. Both nickel and cobalt are determined in the form of their DMG (dimethylglyoxime) complex.
This method had been proven to be reliable with the mercury electrode, meaning that this application can be effectively transferred to a mercury-free electrode as well. This method can comfortably comply with legal requirements thanks to a detection limit of 1 µg/L via the 946 Portable Analyzer, and 0.2 µg/L via the 884 Professional VA lab instrument.
The recovery for 1 µg/L Ni in a standard solution is around 99% (mean of 10 determinations), with a relative standard deviation of 5%.
Chromium(VI) in Drinking Water
The danger of chromium(VI) in drinking water was highlighted in the year 2000 in the movie Erin Brockovich, starring Julia Roberts. The movie’s plot is based on a true story, which took place in the small community of Hinkley, California.
The local energy provider had contaminated the groundwater with the carcinogenic hexavalent chromium, attempting to cover up the incident. Residents suffered from an increased number of health problems including tumors, and eventually, these were traced back to the contaminated drinking water.
Environmental contamination with Cr(VI) is generally the result of improper process handling in industrial applications, particularly waste dumped from galvanic chromium plating. The World Health Organization advises a maximum limit of 50 µg/L total chromium in drinking water.
Modifying the scTRACE Gold electrode with an ex-situ plated mercury film, allows Chromium(VI) to be determined as a complex with DTPA (diethylenetriaminepentaacetic acid).
Recovery of a standard containing 30 µg/L Cr(VI) is 115% (mean of 3 determinations), with a relative standard deviation of 2%. The 946 Portable VA Analyzer enables the determination of concentrations down to 2 µg/L Cr(VI). This mobile instrument enables onsite determination, returning results immediately.
Summary
Many other applications are made possible thanks to the scTRACE Gold electrode. Metrohm provides a diverse array of methods for the scTRACE Gold. The table below provides an overview of elements for which methods are available. Metrohm can also provide advice and support in determining an element or specific matrix.

Figure 3. The scTRACE Gold electrode from Metrohm is suitable for trace analysis of several elements in the water. Image Credit: Metrohm AG
Overview: Applications with the scTRACE Gold
| Element |
Application document |
| As(total) |
Application Note V-210 |
| As(III) |
Application Note V-211 |
| Hg |
Application Note V-212 |
| Cu |
Application Note V-213 |
| Pb |
Application Note V-214 |
| Zn |
Application Note V-215 |
| Tl |
Application Note V-228 |
| Fe |
Application Note V-216 |
| Ni, Co |
Application Note V-217 |
| Bi |
Application Note V-218 |
| Sb(III) |
Application Note V-229 |
| Cr(VI) |
Application Note V-230 |
Acknowledgments
Produced from materials originally authored by Barbara Zumbrägel at Metrohm.

This information has been sourced, reviewed and adapted from materials provided by Metrohm AG.
For more information on this source, please visit Metrohm AG.