Sponsored by MerckJan 4 2021
Materials with porous features that have been particularly ordered on the nano-scale have key applications in drug delivery systems, diagnostics, optics, cosmetics, catalysis, coatings, bio-separation, gas-separation and nanotechnology. Nanoporous materials are comprised of a crystalline or amorphous framework with void spaces, which may be cage-type or cylindrical. Most nanoporous materials can be arranged into three key categories microporous, mesoporous and macroporous.1
Microporous materials, including carbons, MOFs, zeolites and amorphous glasses, demonstrate exceptionally narrow pore size distributions between 0.5 – 2 nm.2 These materials can exhibit extreme thermal stability and catalytic activity nifty in cracking processes and they can also act as drying agents, ion exchange media and gas separation materials.
Metal organic frameworks (MOFs) are amongst the fastest growing classes of microporous solids.3 Zeolites and related crystalline molecular sieves have an inherent limit on the dimension of their pores as well as accessibility because of the pore templates available for their synthesis.
Comparatively, macroporous materials such as porous polymer beads with pores sizes that range from 50 to 1000 nm offer easy access to the internal pores at the cost of selectivity. These downsides led to the development of mesoporous materials, which possess an intermediate pore size range between 2 – 50 nm.4
Mesoporous materials have a number of key advantages:
- Simple functionalization strategies with organics
- Narrow pore size distributions and high surface areas (>500 m2/g).
- Biocompatibility and low toxicity.
- Framework/wall substitutions with various metal oxides (MO2) including silica, alumina and titania
Structural Properties and Characterization of Mesoporous Materials
Mesoporous materials that are well-organized can be classified as per their structural dimensions and pore geometry, e.g., either (3D-) cage-type structures or (2D- or 3D-) cylindrical. Cage-type mesocaged solids such as FDU-1(Imm), SBA-1(Pmn) and AMS-8(Fdm) are made up of spherical or ellipsoidal cages that are interconnected 3-dimensionally by smaller cage-connecting windows, which could be used to manipulate the mass transfer of active agents.
In contrast, Cylindrical structures such as MCM-48, AMS-6 (Iad), MCM-41, SBA-15 and NFM-1 (p6mm) possess uniform pore diameter and demonstrate potential towards applications in catalysis, adsorption and as drug delivery vehicles.
Characterization methods such as powder x-ray diffraction, transmission electron microscopy (TEM) scanning electron microscopy (SEM) and N2 adsorption/desorption are frequently used to clarify the structural and textural properties of mesoporous materials such as silica as illustrated in Figure 1.

Figure 1. Scanning Electron Microscopy image of a typical mesoporous silica material. Image Credit: Merck
Powder X-ray diffraction (XRD) is typically used to determine the crystallographic symmetry of material phases at both the nano- and meso-scale. Phase identification for mesoporous materials from powder X-ray diffraction can be complicated as most of the peaks materialize at low angles and may overlap due to closely related short-range order.
Figure 2 exhibits a standard result of the morphology and structural order of the pores inside mesoporous silica. Comprehensive analysis of pore order and symmetry at the mesoscale can be reached utilizing high resolution transmission electron microscopy (HRTEM). HRTEM characterization is vital for acquiring detailed information at these length scales.
Gas adsorption is a complementary technique used to achieve a detailed characterization of porous materials. Adsorption of gases at different relative pressures on the porous solids produces information about properties such as surface area, pore volume and pore size. Figure 3 exhibits a standard gas absorption isotherm.
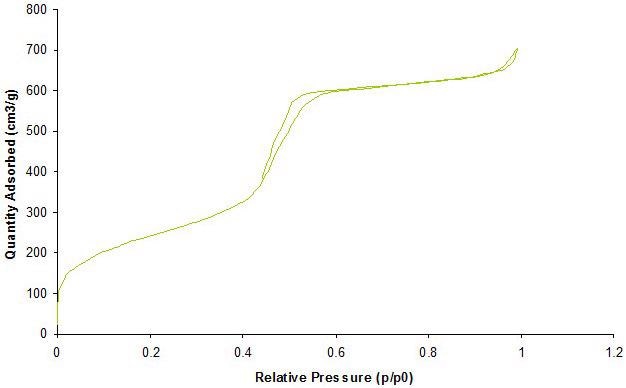
Figure 2. X-ray diffraction of a typical cubic mesoporous silica material. Image Credit: Merck
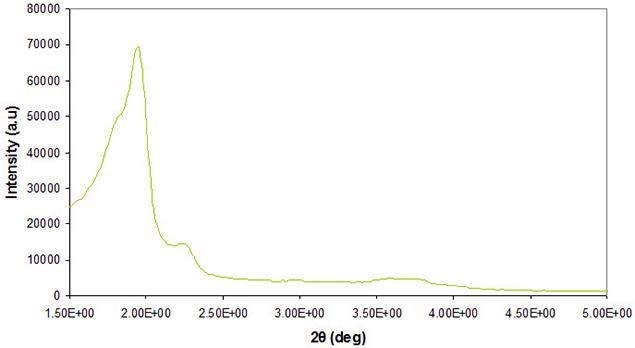
Figure 3. Representative characterization of mesoporous silica nanoparticles by N2-Isotherm corresponding to a surface area 850 m2/g with a pore size of 3.8 nm. Image Credit: Merck
Functional Mesoporous Silicas
Among the most adaptable categories of mesoporous materials are porous silicas which incorporate well defined pore sizes with the well-established biocompatibility of silica in a range of applications.
A principal property of a porous silica material is the capacity to integrate functional organics (R) on the silica wall. Porous silica has a high density of surface silanol (≡Si-OH) groups post-calcination. The reaction of these silanols with different silanes introduces various functional groups (≡Si-R) on the silica framework, which can be utilized to conjugate molecules of interest.5
Properties and Potential Applications of Functionalized Materials
- Loading of nanoparticles including iron oxide, gold, etc.
- Encapsulation of pharmaceutical drugs, proteins and other biological molecules
- Loading of active sites for catalysis
- Adsorbent for gases, ions and molecules
Nanoporous Si modified with three different functional groups are available: Propylamino, Propylcarboxylic Acid and Propylthiol groups.
Applications of Nanoporous Materials in Research and Industry
Drug Delivery Systems
The major challenge when developing drug delivery systems (DDS) is that drug efficacy deteriorates prior to reaching the target, fundamentally due to the excretion of the drug from the body. Additionally, the drug carrier needs to be non-toxic and inert throughout the treatment period. Due to the fact most biological molecules and pharmaceuticals are on the order of a few nanometers, nanoporous silica with a pore size of 2 – 30 nm is of significant importance for such life science applications.6
Catalysis
In catalysis, materials with large surface areas and nanoscale features are utilized to develop highly selective catalysts that limit energy use and the production of waste and pollutants in industrial applications.7 Porous materials, such as zeolites (microporous solids) are broadly used in industry as catalysts and catalyst supports.
Yet, when large molecules are incorporated into a catalyzed reaction, mass transfer inhibits the suitability of zeolite structures. Attempts to enhance the diffusion of reactants to catalytic sites have been resolved by expanding pore sizes to the meso range8. These super-selective catalysts can significantly reduce costs in many industries.
Diagnostics
Mesoporous materials are perfect in diagnostics applications because of their enhanced image contrast and chemical stability. Functional moieties can be united within the pores enabling new potentialities for numerous measurements and detection. Due to the low toxicity of silica based porous materials and their capacity to host a wide range of fluorescent markers, dyes and drugs can be used to monitor and find the location of therapeutic agents and their activity.
Adsorbents
The large surface area of nanoporous materials facilitates their use as adsorbents for a variety of liquids, gases and toxic heavy metals. The uptake of these substances can be significantly increased based on the surface properties (hydrophilicity, hydrophobicity, or functionality), of the mesoporous silica materials. Multiple applications, including the removal of pollutants from water, adsorptive xylene separation, storage of gases (e.g., CO2, H2, O2, CH4, H2S) and separation of biological and pharmaceutical compounds, have been addressed by utilizing mesoporous materials as adsorbents.
Chromatography
The large surface area, pore volume and narrow pore-size distribution of mesoporous silica, makes it an optimal choice for size exclusion chromatography. These materials have been recommended as supports or stationary phases for normal phase High Pressure Liquid Chromatography (HPLC), capillary gas chromatography, size exclusion chromatography, proteomics separations and enantioselective HPLC.
References and Further Reading
- Wan, Y.; Zhao, D. Y. Chem. Rev. 2007, 107, 2821.
- Wright, P. A. (2008) Microporous Framework Solids, RSC Publishing.
- Yaghi, O. M.; Li, G. M.; Li, H. L. Nature 1995, 378, 703.
- Beck, J. S.; Vartuli, J. C.; M.E, L.; C.T, K.; Schmitt, K. D; Chu, C. T. W.; Olson, D. H.; Sheppard, E. W.; McCullen, S. B.; Higgins, J. B.; J.L, S. J. Am. Chem. Soc 1992, 114, 10834..
- Doadrio, J. C.; Sousa, E. M. B.; Izquierdo-Barba, I.; Doadrio, A. L.; Perez-Pariente, J.; Vallet-Regi, M. J. Mater. Chem. 2006, 16, 462.
- Vallet-Regi, M.; Balas, F.; Arcos, D. Angew. Chem. Int. Ed. 2007, 46, 7548.
- Taguchi, A.; Schuth, F. Microporous Mesoporous Mater. 2005, 77, 1.
- Sayari, A.; Chem. Mater. 1996, 8, 1840.

This information has been sourced, reviewed and adapted from materials provided by Merck.
For more information on this source, please visit Merck.