Nickel’s (Ni) corrosion resistance and strength at both high and low temperatures have led to it being one of the most frequently utilized metals.
Nickel is typically used in steel production, but it also sees routine use as a component in a range of different alloys. Nickel is also a popular material choice in plating, electronics and rechargeable batteries.
As it is used in such a diverse array of applications, there is an ongoing need for nickel of different purities. For example, some applications necessitate the use of high-purity nickel, while others will find lower-grade nickel to be sufficient.
The London Metal Exchange (LME) provides specifications detailing a range of purities for various metals.
The study presented here showcases the use of PerkinElmer’s Avio® 550 Max ICP optical emission spectrometer (ICP-OES) for the analysis of contaminants in nickel. The study uses the LME’s ‘Special Contract Rules for Primary Nickel’1 as a guideline for the use of analytes and required concentrations.
Experimental
Samples
Analyses in this study were conducted in 1% solutions of Ni to simulate 100x dilution with 5% nitric acid (v/v). Elemental spikes were added to the 1% Ni solution to confirm accuracy.
These were set at the levels defined in the ‘Special Contract Rules for Primary Nickel’1 according to the ASTM Standard Specification for Nickel (99.80%).
Analyses were conducted and evaluated against external calibration curves comprised of standards prepared in 5% HNO3 at 0.25, 0.5 and 1.0 ppm.
Table 1. Avio 550 Max ICP-OES Instrumental Parameters and Conditions. Source: PerkinElmer
| Parameter |
Value |
| Nebulizer |
MEINHARD® K-1 |
| Spray Chamber |
Baffled glass cyclonic |
| RF Power |
1500 W |
| Injector |
2.0 mm ceramic |
| Plasma Gas Flow |
8 L/min |
| Aux Gas Flow |
0.2 L/min |
| Nebulizer Gas Flow |
0.60 L/min |
| Torch Position |
-4 |
| Sample Uptake Rate |
1.0 mL/min |
| Sample Uptake Tubing |
Black/Black (0.76 mm id) |
| Internal Standard Tubing |
Green/Orange (0.38 mm id) |
| Drain Tubing |
Red/Red (1.14 mm id) |
| Replicates |
3 |
| Plasma View |
Axial |
| Integration Time |
5 sec (min and max) |
| Integration Range |
0.5 – 2 sec |
Table 2. Elements and Wavelengths. Source: PerkinElmer
| Element |
Wavelength (nm) |
| As |
188.979 |
| Bi |
306.766 |
| Co |
238.892 |
| Cu |
324.752 |
| Fe |
238.204 |
| Mn |
259.372 |
| P |
178.221 |
| Pb |
283.306 |
| S |
181.975 |
| Sb |
206.836 |
| Si |
288.158 |
| Sn |
283.998 |
| Zn |
206.200 |
| Sc (int std) |
361.383 |
Instrumentation
The Avio 550 Max fully simultaneous ICP-OES was used to perform all analyses. Table 1 shows the instrument parameters, while Table 2 shows the analytes and wavelengths used.
Standard sample introduction parameters and configuration were employed with every analysis, with the torch position set to -4.
It should be noted that the simultaneous analysis capabilities of the Avio 550 Max coupled with its low argon consumption (9 L per minute total) affords users excellent cost savings, particularly given the high cost of argon.
Table 3. London Metal Exchange Impurity Levels in 99.80% Ni. Source: PerkinElmer
| Element |
Specification
(wt %) |
Concentration in 1%
Ni Solution (mg/L) |
| As |
0.005 |
0.5 |
| Bi |
0.005 |
0.5 |
| Co |
0.15 |
15 |
| Cu |
0.02 |
2 |
| Fe |
0.02 |
2 |
| Mn |
0.005 |
0.5 |
| P |
0.005 |
0.5 |
| Pb |
0.005 |
0.5 |
| S |
0.01 |
1 |
| Sb |
0.005 |
0.5 |
| Si |
0.005 |
0.5 |
| S |
0.01 |
1 |
| Sn |
0.005 |
0.5 |
| Zn |
0.005 |
0.5 |
Results and Discussion
Table 3 provides the ASTM Standard Specification for Nickel at 99.80% weight percent.1 It also displays concentrations in a 1% Ni solution, which is equivalent to a 100x dilution.
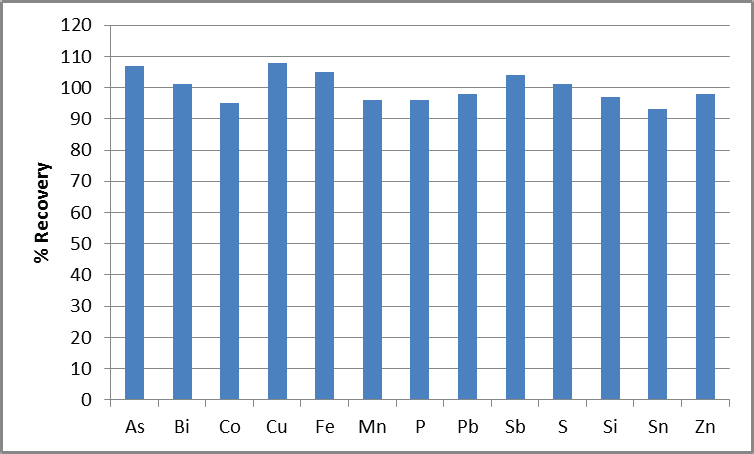
Figure 1. Recovery of analyte spikes in 1% Ni. Spike concentrations are shown in Table 3.
Figure 1 displays recoveries for a 1% Ni solution spiked with elements at the listed concentrations, with all recoveries found to be within 10%. This confidently confirms the instrument’s capacity to accurately measure these elements at low concentration levels.
Significant spectral interferences arose from the Ni matrix, impacting the measurement of Bi and Sn. This interference was removed via the application of a multicomponent spectral fitting (MSF) model2 to these elements, allowing for accurate measurements at the required levels.
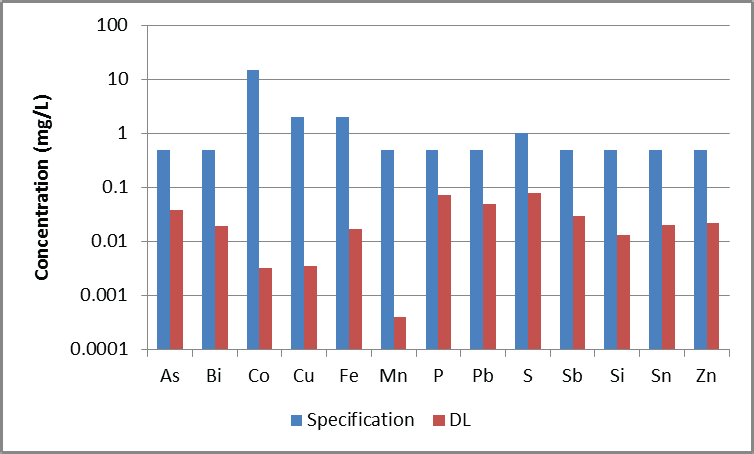
Figure 2. Detection limits in 1% Ni, along with the specification limits for 99.80% Ni, accounting for 100x dilution.
It was determined that detection limits in solution were 3x the standard deviation of seven consecutive measurements of 1% Ni. Detection and specification limits are shown in Figure 2, accounting for the 100x dilution.
With the instrument and method’s accuracy established, stability was assessed via the monitoring of the internal standard signal of 1% Ni over a 6-hour analysis period.
Figure 3 plots the results of this analysis, highlighting a variation of less than 5% when normalized to the initial reading. These results confirm the stability of the methodology.
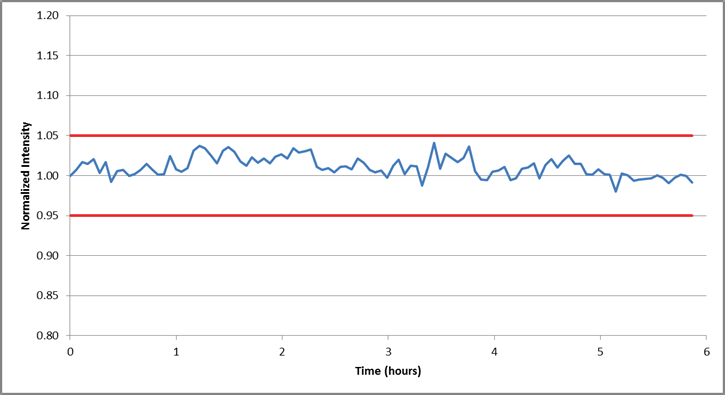
Figure 3. Internal standard (Sc) stability over a 6-hour analysis of 1% Ni solution. All data is normalized to the first reading.
Conclusions
The study presented here successfully highlights the PerkinElmer Avio 550 Max fully simultaneous ICP-OES’s capacity to analyze solutions of 1% nickel for elements at the levels defined by the London Metal Exchange.
It was possible to mitigate the effects of spectral interferences from high matrix samples via MSF, enabling all of the specified elements to be measured at their defined concentrations.
References
- "Special Contract Rules for Primary Nickel", The London Metal Exchange.
- "Multicomponent Spectral Fitting", Technical Note, PerkinElmer, 2016.
Consumables Used
Table 4. Source: PerkinElmer
| Component |
Part Number |
Sample Uptake Tubing, Black/Black
(0.76 mm id), PVC |
N0777043 (flared)
09908587 (non-flared) |
| Drain Tubing, Red/Red (1.14 mm id), PVC |
09908585 |
Internal Standard Tubing, Orange/Green
(0.38 mm id), PVC |
N0773111 (flared) |
| Antimony Standard, 1000 mg/L |
N9300207 (125 mL)
N9300101 (500 mL) |
| Arsenic Standard, 1000 mg/L |
N9300180 (125 mL)
N9300102 (500 mL) |
| Bismuth Standard, 1000 mg/L |
N9303761 (125 mL)
N9300105 (500 mL) |
| Cobalt Standard, 1000 mg/L |
N9303766 (125 mL)
N9300113 (500 mL) |
| Copper Standard, 1000 mg/L |
N9300183 (125 mL)
N9300114 (500 mL) |
| Iron Standard, 1000 mg/L |
N9303771 (125 mL)
N9300126 (500 mL) |
| Lead Standard, 1000 mg/L |
N9300175 (125 mL)
N9300128 (500 mL) |
| Manganese Standard, 1000 mg/L |
N9303783 (125 mL)
N9300132 (500 mL) |
| Phosphorus Standard, 1000 mg/L |
N9303788 (125 mL)
N9300139 (500 mL) |
| Scandium Standard, 1000 mg/L |
N9303798 (125 mL)
N9300148 (500 mL) |
| Silicon Standard, 1000 mg/L |
N9303799 (125 mL)
N9300150 (500 mL) |
| Sulfur Standard, 1000 mg/L |
N9303796 (125 mL)
N9300154 (500 mL) |
| Tin Standard, 1000 mg/L |
N9303801 (125 mL)
N9300161 (500 mL) |
| Zinc Standard, 1000 mg/L |
N9300178 (125 mL)
N9300168 (500 mL) |
| Autosampler Tubes |
B0193233 (15 mL)
B0193234 (50 mL) |
Acknowledgments
Produced from materials originally authored by Aaron Hineman and Ken Neubauer from PerkinElmer, Inc.

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer.
For more information on this source, please visit PerkinElmer.