A range of production processes frequently employ acid mixtures comprised of two or three acids; for example, nitric acids, sulfuric acids and hydrochloric acids.
In order to use these acids safely and effectively, it is important to have accurate knowledge of each single acid component’s content. This is also crucial for workflow optimization.
One of the common issues with the titration of acid mixture is that this process does not typically lead to more than one equivalence point. It is, therefore, not always possible to determine the content of every acid using a single titration in an aqueous solution.
Depending on the strength of the acid in the specific solvent, this may exhibit a potential jump at the equivalence point in the titration curve during titration.
This can be overcome by optimizing the choice of the solvent and of the titrant. This Guide covers selected applications and background information for the simultaneous titration of the acid components in a mixture with Titrator Excellence T5, T7 and T9. The Guide consist of three parts:
- Selected titration applications: The results for typical acid mixtures such as HCl/HNO3 and CH3COOH/H3PO4/HNO3 are displayed. The applications have been developed by the Market Support Group Analytical Chemistry, and they are available from the Expertise Library on www.mt.com.
- Question on acid/base titrations: Relevant background information is presented using frequently asked questions (FAQs).
- Non-aqueous acid/base titrations: Fundamental basics on acids and bases in aqueous and non-aqueous solvents are outlined in detail. This section includes recommendations on choosing suitable solvents and titrants for differentiation of each single acid (base) component of a mixture during titration.
This Guide will support work on content determination in acid mixtures.
Titration’s Role in Electroplating
Acid mixtures are frequently employed in electroplating baths as a means of treating the surface of metal parts to enhance their resistance to corrosion and abrasion.
These mixtures are generally comprised of strong acids such as hydrochloric acid (HCl), hydrofluoric acid (HF), sulfuric acid (H2SO4) and phosphoric acid (H3PO4). These mixtures also include weak acids such as acetic acid (CH3COOH).
As electroplating processes are impacted by acid content, it is important to monitor and appropriately analyze this to maintain the processes’ efficiency and efficacy.
Understanding Titration
Titration is an easy-to-use, straightforward and cost-effective means of determining the acid content determination in various solutions.
This process involves the addition of a strong alkaline solution to the sample - for example, sodium (NaOH) or potassium hydroxide (KOH) - with the potential difference monitored with a potentiometric sensor (a pH electrode) throughout the process.
This will result in the production of a titration curve, allowing the process to be visualized and explored. Alkaline titrant solution will be added to the sample until a clear potential change is observed. This can also be understood as a potential jump.
Should a single acid be present in the sample solution, the titration curve will exhibit a clear S-shaped profile. The titration’s equivalence point is located at the inflection point of the curve.
Effective Titration of Acid Mixtures
Titration of an acid mixture containing a number of different acids would be expected to yield a titration curve with as many potential jumps as there are different acids in the sample solution.
This can be seen in the example image below which represents an acid mixture containing hydrochloric acid, acetic acid and ammonium chloride. This is also noted as - HCl: CH3COOH: NH4Cl.
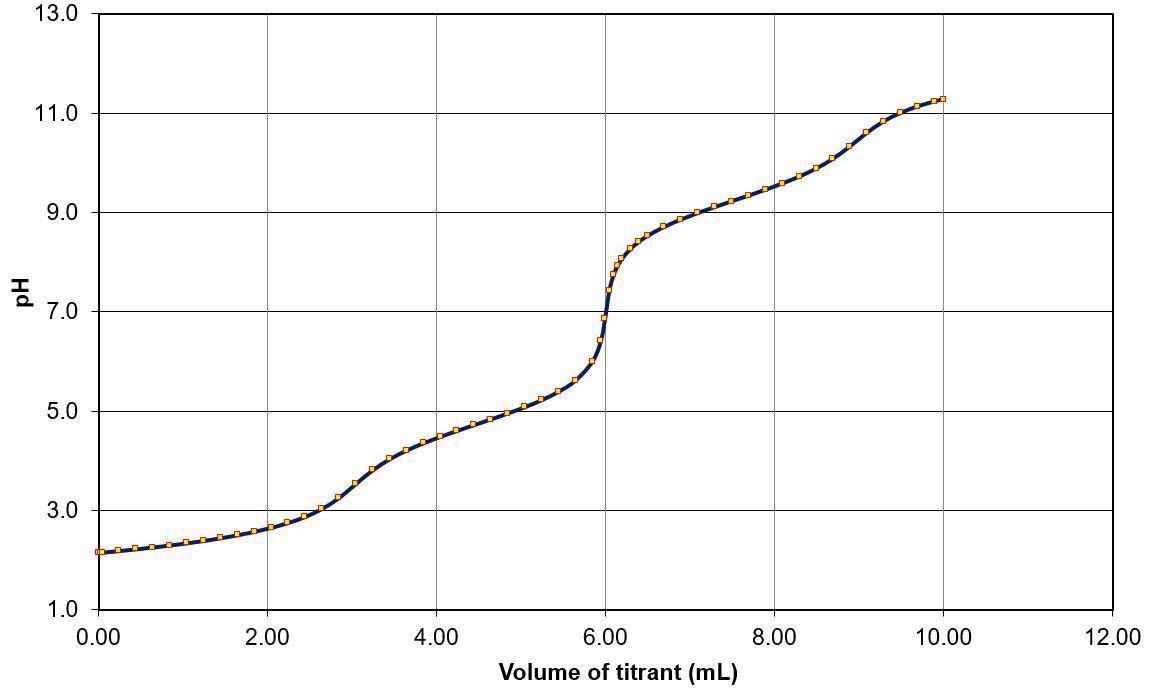
Figure 1. Simulated titration curve of 3 mL each of 0.1 M HCl (strong acid), 0.1 M CH3COOH (weak acid strength), and 0.1 M NH4Cl (very weak acid) in water with 0.1 M NaOH (CurTitPot, http://www.iq.usp.br/gutz/Curtipot_.html). Image Credit: Mettler-Toledo - Titration
In the example presented here, every acid has an identical concentration (0.1 M) and sample size. This mixture has been diluted with 50 mL of deionized water prior to being titrated with a strong base – for example, sodium hydroxide - having the same concentration (0.1 M NaOH).
The titration curve exhibits a total of three potential jumps at 3, 6 and 9 mL of titrant consumption. It should be noted that each of these potential jumps reflects the equivalence point (EQP) of the corresponding neutralization reaction.
Using the determined EQP, it is possible to calculate the content of each acid present in the mix.
Despite these findings, it is still necessary to ascertain which potential jump corresponds to the acids present.
The sequence of the three potential jumps in the curve is defined by the acids’ strengths. The strongest acid is neutralized first, with neutralization of the weaker acids occurring in sequence once the stronger acid has been neutralized. Essentially, this means that acids in a mixture are neutralized one after the other, in strength order.
The first potential jump HCl: CH3COOH: NH4Cl mixture corresponds to the strongest acid, HCl. Acetic acid is then neutralized (the second jump), while the third jump corresponds to NH4Cl as the weakest acid.
Figure 2. HCl is the strongest acids compared to acetic acid and ammonium chloride. Image Credit: Mettler-Toledo - Titration
The Acid Dissociation Constant: pKa
An acid’s strength is quantified by the acid dissociation constant (Ka). Its strength is therefore specified using the pKa value - the negative logarithm of the dissociation constant (Ka) of an acid (HA):
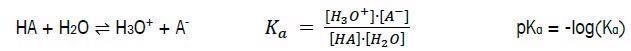
The pKa values for a range of acids in aqueous solutions are shown below:
Table 1. Acid strength of selected acids: The lower the pKa value, the stronger the acid, as indicated by the arrow below procedure. Source: Mettler-Toledo - Titration
| Acid |
pKa (25 °C)
- D.W. Oxtoby et al., "Principle of Modern Chemistry", 3rd ed., Saunders Publ., 1996
- * Wikipedia
|
Acid strength |
| HCl |
-7 |
 |
| H2SO4 |
-3 pKa1 |
| 1.92 pKa2 |
| HNO3 |
-1.3 |
H2C2O4
(Oxalic acid) |
1.25 * pKa1 |
| 4.14 * pKa2 |
| H3PO4 |
2.12 pKa1 |
| 7.21 pKa2 |
| 12.67 pKa3 |
| HF |
3.18 |
CH3COOH
(Acetic acid) |
4.75 |
CH3COOOH
(Peracetic acid) |
8.2 * |
NH4Cl
(Ammonium chloride) |
9.24 * |
Differentiation of Acids in Mixtures
The acid mixture 0.1 M HCl: CH3COOH: NH4Cl represents a 1:1:1 v/v in an aqueous solution. This is an ideal mixture for the determination of the content of each acid via titration for a number of reasons:
- There are significant differences (∆pKa) in acid strengths; for example, ∆pKa (HCl - CH3COOH) > 11 and ∆pKa (CH3COOH - NH4Cl) > 4
- Each acid exhibits the same concentration of 0.1 M
- The mixture is comprised of equal amounts (3 mL) of each acid
While this example composition represents the ideal, real sample mixtures from working electroplating baths tend to be comprised of acids with similar strengths, varying amounts and different concentrations.
Titration curves for these real-world acid mixtures tend to overlap as some of the dissociation steps collapse into a single step, prompting a failure in the resulting titration curve of an acid mixture to indicate the potential jump of each acid.
This means that it is only possible to use titration to properly evaluate the potential jumps of all acid components in an aqueous mixture:
- The acids’ pKa values are different by at least four or five units
- The acids’ concentrations and amounts are close to equal
When performing the titration of acid mixtures in aqueous solutions, it is, therefore, necessary to perform an initial check of their pKa value.
When considering the HCl: CH3COOH: NH4Cl mixture presented in the earlier example, this is the reason that the three potential jumps are visible in the titration curve (Figure 1).
This would be unlikely to be achievable outside of an example mixture because the pKa values of acids analyzed in real-world applications are generally too close to one another.
It is still possible, however, to differentiate the different potential jumps in a titration curve to some degree.
This can be achieved by replacing the water with an appropriate organic solvent; for example, acetone, 2-propanol, ethanol, methanol or mixtures of these solvents. The addition of an inert salt can also be used to modify the ionic strength of the aqueous solution; for example, potassium chloride (KCl) or sodium chloride (NaCl).
Each of these approaches results in a shift in pKa values, increasing the difference between these. For instance, it is possible to dissolve the sample in acetone and titrate this with either tetraethylammonium or tetramethylammonium hydroxide in 2-propanol.
It is also important to employ appropriate evaluation parameters when calculating method functions. Ensuring these are in place will further support the determination of the equivalence point (EQP) for each individual anticipated potential jump.
The table below provides examples of acid mixtures and polyprotic acid titrations.
Table 2. Simultaneous titrations of acid mixtures and of polyprotic acids. Source: Mettler-Toledo - Titration
Acid mixtures /
Polyprotic acids |
Titration |
Solution |
Polyprotic acids
e.g. :
H2SO4
H3PO4
H2C2O4 |
Titration to all EQPs |
- Identify the pKa value of each dissociation step
- Verify if the values are different by more than approx. 4-5 pKa units to be visible
- make sure the pKa values are smaller than the autodissociation constant of water pKw = 14
- If YES: Try a titration in aqueous solutions
|
| H3PO4 |
Titration to 3 EQPs |
- Sample dissolved in saturated NaCl solution
- Two titration (EQP) functions with two different threshold values
|
| HNO3 : HF : CH3COOH |
Differentiation of 3 EQPs |
- Sample dissolved in acetone: 2-propanol 1:1 v/v
|
| CH3COOH : H3PO4 : HNO3 |
Content determination by evaluation
of 3 EQPs in aqueous solution |
- The differences between the dissociation steps are large enough to get a potential jump.
- Identify the pKa value of each dissociation step
- Verify if the pKa difference is more than 4-5
- Verify if pKa values are below/very close to the autodissociation constant of water pKw = 14
|
CH3COOH : H3PO4 : HNO3
Large excess of H3PO4 |
Evaluation of 3 EQPs at specific potential values |
- Identify the pKa value of each dissociation step
- The first derivative curve shows small and almost identical peaks for the first two EQPs
- These peaks cannot be evaluated using specific threshold values since very small and close to each other
|
CH3COOH : H3PO4 : HNO3
Concentrations below 0.3% |
Evaluation of 3 EQPs with potential ranges |
- Identify the pKa value of each dissociation step
- The first derivative curve shows two small peak values for the first two EQPs
- These are evaluated with a potential range
|
When working with the titration of acid mixtures, it is advisable to be aware of a number key points. The mixture’s composition should be considered; for example, which acids are present in the mixture. The quantity of each acid present in the mixture should be considered, as well as any additional components.

 Download the full Titration guide
Download the full Titration guide
The acid dissociation constants (pKa values) of the acid mixtures should be determined. If the difference between the acids’ pKa values is greater than four or five, the mixture may be titrated in water. If these differences are less, it will be necessary to titrate the mixture using another solvent.

This information has been sourced, reviewed and adapted from materials provided by Mettler-Toledo - Titration.
For more information on this source, please visit Mettler-Toledo - Titration.