Calorimetry measures the amount of heat released or absorbed during a chemical reaction. It is a beneficial and powerful tool that accurately evaluates chemical processes, helps researchers assess potential risks, and provides information on non-scalable conditions.

Large iron heat exchanger. tank, reactor, distillation column in thermal insulation of fiberglass and mineral wool of galvanized steel in the industrial refinery petrochemical plant shop. Image Credit: H.E.L Group
It is vital to assess any potential risk during a chemical reaction, such as increases in pressure, temperature, and decomposition of the reactants, which may affect analytical results and product quality.
The Dangers of Runaway Reactions
Decomposition can occur when a reactant breaks down into two or more by-products. This is especially common when the reactant is highly unstable. The situation can be exacerbated if heat accumulates during the reaction over time, increasing temperature.
This raises the risk of a runaway reaction, which can have severe consequences for operator safety and damage vital equipment. A runaway reaction is a hazardous positive feedback loop that accelerates an unstable exothermic reaction.
During a runaway reaction, further energy release increases the reaction rate. Monitoring reactions to ensure this does not happen is essential. Between 1984 and 2019, there were 217 recorded incidents linked to runaway reactions in China and the EU.
In the US, The US Chemical Safety and Hazard Investigation Board reported at least 167 major incidents. The UK Health & Safety Executive receives reports, on average, every two to three weeks on accidents involving runaway chemical reactions.
Understanding Chemical Reaction Risks and Safety
The maximum temperature of the synthesis reaction (MTSR) is a fundamental parameter in developing safer reactions. This is defined as reaction temperatures + adiabatic temperature rise.
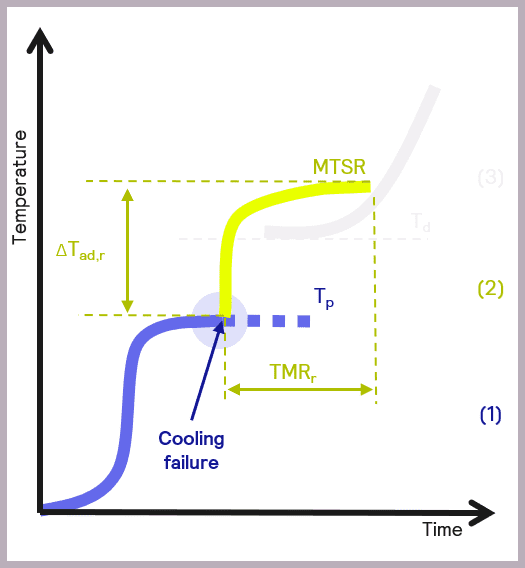
Image Credit: H.E.L Group
In calorimetry, adiabatic temperature rise is a fundamental concept related to temperature changes that occur due to chemical reactions in the reactor. Adiabatic temperature refers to temperature change when there is no gain or loss of heat.
Aside from high MTSR values and their inherent risk to workers, equipment, and resultant physical processes, such as pressure rise and evaporation in reactors that carry a risk of explosion, undesired chemical reactions are another key concern.
High temperatures are the perfect conditions for decomposition reactions and can cause the proliferation of flammable, toxic, or explosive substances.
How is Calorimetry Suited for Monitoring Runaway Reaction Risk?
Calorimetry is highly suited to this purpose. Heat release from chemical reactions has a linear relationship with reactant mass. By simulating reactions at a smaller scale, calorimetry improves the identification of potential risks.
By closely monitoring the reaction, heat flow and temperatures can be observed and analyzed to understand several reaction kinetics and behavior better.
These kinetics and behaviors include velocity at which the reaction occurs, reagent accumulation, and unexpected or abnormal behaviors in the head flow, indicating secondary reactions. This information allows researchers and engineers to design improved control measures that reduce risks.
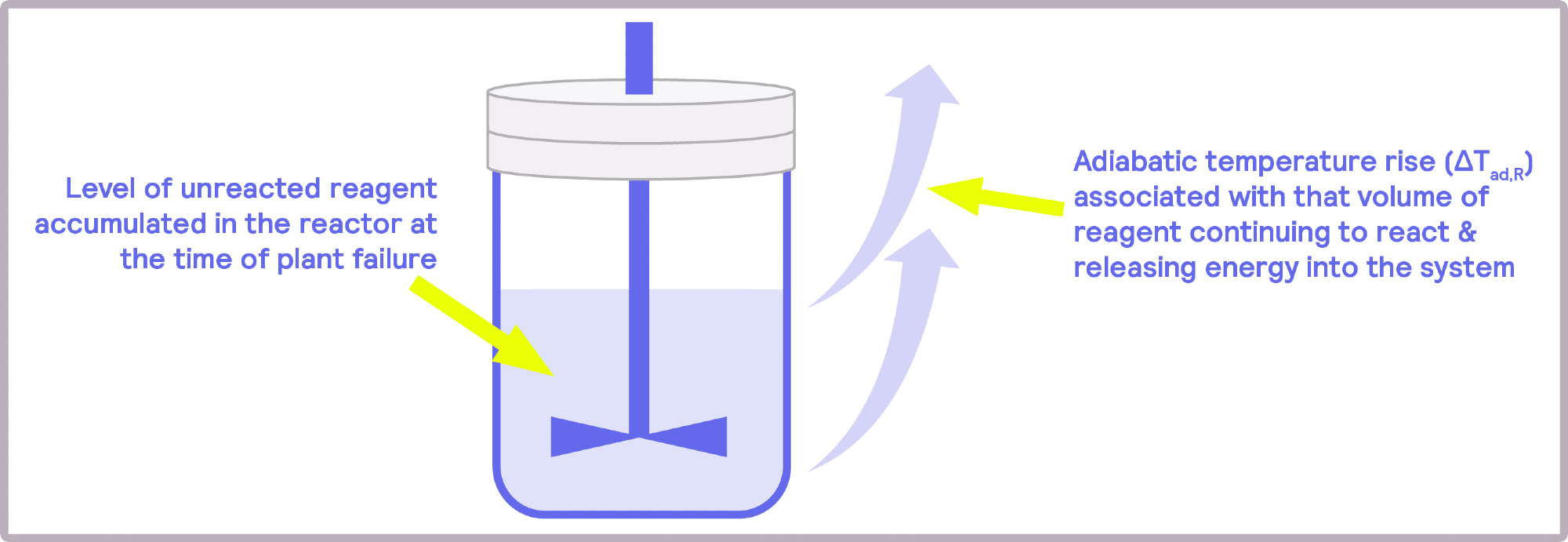
Image Credit: H.E.L Group
Towards Safer Processes
Calorimetry’s ability to mimic process conditions gives enhanced insight into the chemical reaction. Measures can be put in place based on the information and data gathered.
Different scenarios call for different solutions: for instance, an exothermic reaction wherein MSTR values are higher than the decomposition temperature of some of its components may require cooling to reduce the risk of accidents.
Another critical issue is the potential accumulation of heat post-feed process. Batch reactors can change to semi-batch reactors, where instead of all reactants being combined, one of the reactants can be progressively supplied. This adjustment to the reaction avoids reactant accumulation and sudden temperature increases.
By adjusting the feed rate in semi-batch reactions, the reaction can progress more slowly and efficiently, preventing the sudden accumulation of reactants, which can be highly dangerous.
Learning From Calorimetry
Increased production is a hallmark of industrial and social progress. However, much can be learned from basic chemistry, with calorimetry being a prime example of a technique that can provide information and benefit safer and more efficient production processes.
Understanding if a reaction is exothermic helps process engineers design improved mechanisms, keeping temperature increases to safe levels. Predicting undesired secondary reactions also avoids sudden over-pressurization, which can lead to fatal explosions. Energy accumulation can be reduced by fine-tuning reactors.
Calorimetry is a process that can be applied in multiple industrial sectors to reduce risks and help protect workers and avoid financial loss associated with major incidents.

This information has been sourced, reviewed and adapted from materials provided by H.E.L Group.
For more information on this source, please visit H.E.L Group.