Sponsored by HORIBAReviewed by Olivia FrostJan 20 2026
Selecting the right protecting group is essential for the success of organic syntheses and the manipulation of polyfunctional molecules, as it can prevent the formation of undesired side products and reactions.
Photoremovable protecting groups have several advantages, such as mild deprotection conditions and compatibility with acid- and base-sensitive groups.
They are used in controlled molecule release in materials science, caging and releasing biologically important compounds, and synthetic organic chemistry.
Photocleavage Reaction
Typically, the photocleavage reaction involves the formation of an ion pair from the excited ester, which can either recombine or dissociate to yield the corresponding alcohol, as illustrated in the simplified scheme for an amino acid-heterocycle system in Figure 1.
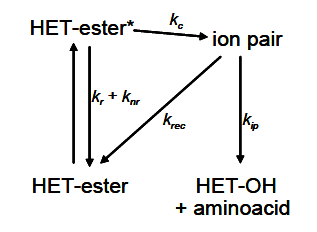
Scheme I. Simplified photocleavage scheme. Image Credit: HORIBA
In some cases, there can be a mixture of fluorescing species present, and these can each have their own characteristic decay time and spectrum.
To get the best results, it is desirable to have control over the cleavage conditions, including good photochemical yield, cleavage rate, and selectivity through the best choice of wavelength.
For biological applications, the best wavelengths to use are over 350 nm, as this avoids cell damage caused by UV light.
This approach also allows for two-photon excitation, which is beneficial for biological applications and enhances selectivity, as only a small (femtoliter) volume will be excited.
Time-Resolved Fluorescence Measurements
Since the selected system is likely to contain several spectrally overlapping species upon photoexcitation, a simple steady-state measurement cannot adequately characterise it or reveal the underlying dynamics.
A time-resolved approach, such as recording the time-resolved emission spectrum (TRES), is more suitable for this purpose. Acquiring TRES data requires an emission monochromator.
A suitable system for this purpose is the HORIBA FluoroCube, as depicted in Figure 2.
Fig 1. FluoroCube-01 with DeltaDiode excitation. Image Credit: HORIBA
Measurements are usually taken by stepping the monochromator through fixed wavelength intervals, collecting time-resolved decays at each step either for set time intervals or until a specified peak count is reached. The fixed time interval mode should be used if intensity information is required.
Acquiring the instrumental response function (IRF or prompt) enables further analysis through deconvolution. As shown schematically in Figure 3, decay-associated spectra can also be obtained.
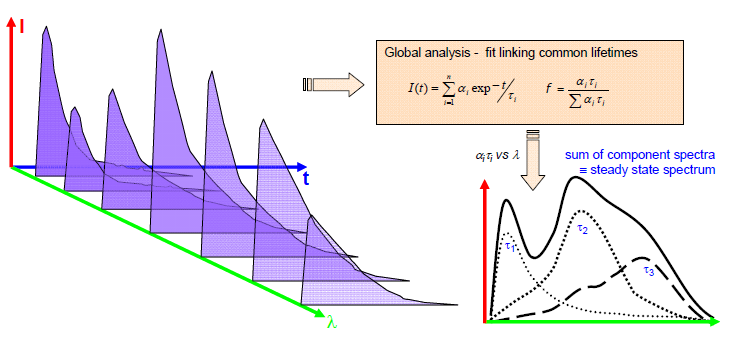
Fig 2. Scheme for obtaining decay associated spectra. Image Credit: HORIBA
This approach facilitates the correlation of spectra with the different decay times, thereby aiding in the elucidation of the species present.
Photocleavage of Novel Amino Acid Ester Derivatives
This example is based on the work of Costa and Gonçalves (see reference), using the compounds depicted in Figure 4.
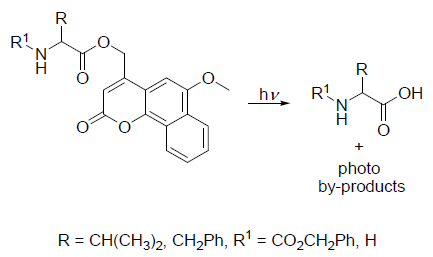
Scheme II. Compounds used and expected reaction. Image Credit: HORIBA
Time-resolved decays, measured using a FluoroCube, indicate that the substituent moieties on the amino acid influenced the decay kinetics, as demonstrated in the data presented in Figure 5.
This figure illustrates how changes in substituents influence time-resolved fluorescence behaviour.
It also includes analysis of a model compound representing the photocleaved HET-OH moiety. A major advantage of time-resolved fluorescence is its ability to provide rapid, qualitative insights into dynamic processes.
The top panel shows clear differences in decay kinetics, while the subsequent panels present the decay-associated spectra (DAS) calculated from the TRES data. Summing the individual DAS reconstructs the overall steady-state spectrum.
These data facilitate comparisons with model compounds and assist in the assignment of species. Notably, the bottom panel reveals that the DAS for the longer-lived decay closely resembles the spectrum of the model compound.
Rate constants can be estimated from the lifetime values, shown in Figure 1, highlighting the utility of time-resolved fluorescence techniques in this field of application.
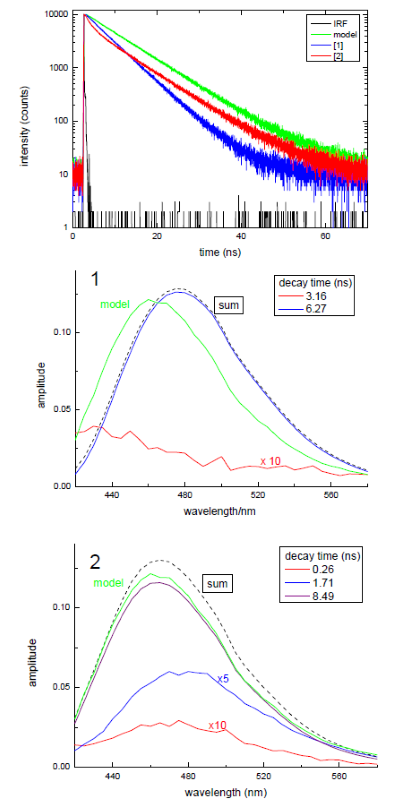
Fig. 2. Example data from time-resolved lifetime and DAS analysis of compounds shown in Scheme II. Image Credit: HORIBA
References and Further Reading
- Piloto, A.M., et al. (2011). Photolysis at long wavelengths of amino acid ester derivatives based on 4-methyl-6-methoxy-2-oxo-2H-naphtho[1,2-b]pyrans. University of Minho. (online) https://doi.org/1434-193X.

This information has been sourced, reviewed and adapted from materials provided by HORIBA.
For more information on this source, please visit HORIBA.