The growing demand for portable electronic devices and electric vehicles (EV) has increased the need for rechargeable batteries. Lithium-ion batteries (LiBs) are the current state of the art in this field, with high energy density, low self-discharge rate, and excellent cycle durability.
As this demand for LiBs increases production rates, maintaining quality standards throughout production is essential. Consequently, analytical techniques and physical methods play an indispensable role in the manufacturing process when characterizing LIBs.
Water testing in LiBs is crucial for quality control, as when water reacts with the electrolyte, it produces harmful degradation products that impair battery performance. Karl Fischer (KF) titration is the generally preferred method for measuring water content in the main components, cathode, anode, and electrolyte, before they are assembled into the battery housing.
In addition to water, hydrofluoric acid (HF), a harmful degradation product of LiPF6 and possibly other fluorinated lithium compounds, can be evaluated via acid-base titration using sodium hydroxide as the titrant, or through precipitation titration of fluoride ions.
Beyond titration methods for water and HF testing, multiple titration applications are available for the characterization of active electrode materials, along with UV/Vis spectro-photometric techniques, density measurements, refractive index, softening point, pH value, and solution conductivity.
METTLER TOLEDO has an extensive portfolio of analytical solutions for the content determination and characterization of lithium-ion battery components. This Competence Guide presents a selection of various analytical techniques, without starting from the basics. In addition to a brief overview, this guide focuses on the following methods:
- Water content by Karl Fischer Titration: Water reacts with the liquid electrolyte in a lithium-ion battery to form aggressive degradation products that affect all components, making comprehensive water analysis essential for quality control.
- Titrations and direct measurements: Metal ion contents such as cobalt, nickel, manganese, and copper can be calculated using titration methods, alongside direct potentiometric measurements for, e.g., fluoride content determinations.
- UV/Vis spectrophotometry: Primary applications include color measurement and metal ion content determination.
- Density measurements: Characterization of components and raw materials in LiB production includes determining the density of liquid substances and electrolytes.
- Multiparameter workflow: Multiple methods can be used in combination and fully automated within a single analytical workflow: density, refractive index, and color of liquid samples are determined in a single run. In addition, pH and conductivity measurements can be performed and automated within the same workflow.
1. Overview
1.1. Motivation
Digital devices, including smartphones, tablets, and laptops, have become indispensable. At the same time, the shift from fossil fuels to renewable energy sources, such as electricity, is overhauling traffic and transportation systems.
However, digitalization and mobility require reliable, durable, and sustainable electricity generation for seamless operation. Accumulators and batteries could be an effective solution.
Among the various types of batteries available for energy supply, the lithium-ion battery (LiB) is an optimal and reliable solution.
What makes the lithium-ion battery so attractive for these applications?
There are four primary advantages:
- These battery types are rechargeable,
- They can be used in various ways,
- Lithium-ion batteries demonstrate exceptional performance, and most importantly
- They can be miniaturized.
1.2. Working Principle of Lithium-ion Batteries
Examination of their structure reveals they consist of just four components: the anode, the cathode, the separator, and the electrolyte. In a lithium-ion battery, electrochemical oxidation of the materials occurs at the anode, while electrochemical reduction takes place at the cathode.
A porous polymer membrane separates the anode and the cathode to prevent a short circuit. The electrolyte is the final component, usually a conductive salt solution, that facilitates the transport of positively charged lithium ions between the anode and the cathode during the discharge and charging processes (Figure 1):
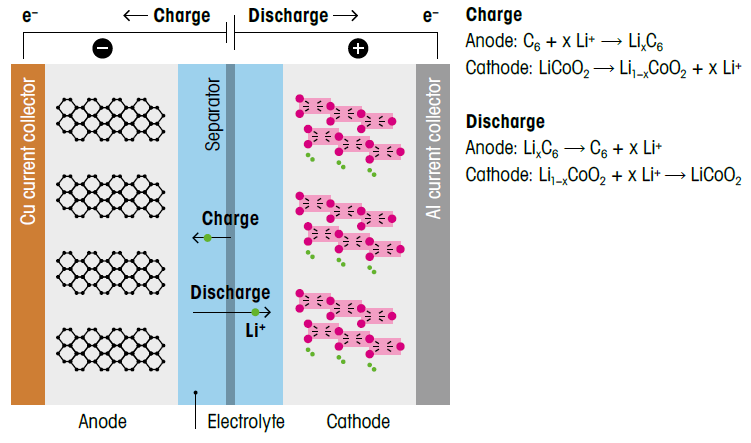
Figure 1. A lithium-ion battery mainly consists of the anode (negatively charged), the cathode (positively charged), the separator, and the electrolyte solution to ensure ionic conductivity between the two compartments. Both electrochemical oxidation and reduction of materials at the anode and the cathode are shown, respectively. Image Credit: Mettler-Toledo - Titration
Understanding all the components is important for selecting the most suitable analytical method for material analysis and characterization. The chemical composition of the components reveals the presence of metals, graphite, polymer materials, and organic solvents.
For example, in addition to lithium, the battery contains metals such as cobalt, titanium, iron, aluminum, copper, manganese, and nickel. The separator is usually made of polypropylene or polyethylene, while the electrolyte typically consists of a solution of LiPF6 in ethylene carbonate and/or ethyl methyl carbonate, with vinylene carbonate serving as a stabilizer.
1.3. Analytical Techniques at a Glance
An overview of the analytical methods offered by METTLER TOLEDO is presented in Table 1:
Table 1. Analysis methods for the main components of lithium-ion batteries. Source: Mettler-Toledo - Titration
| Components |
Battery materials |
Analysis |
Instruments |
| Anode |
- Graphite, petroleum coke
- Lithium titanate, LTO (Li4Ti5O12)
- Solvents for the anode production
(NMP: N-Methyl-2-Pyrrolidone)
|
- Water content determination
- Karl Fischer (KF) Titration
- Electrode slurry
- Softening Point determination
|
KF Titrators
MP/DP instruments |
| Electrolyte |
- Salt: Lithium hexafluorophosphate, LiPF6
- Organic solvents e.g.
- Ethylene carbonate, EC, (CH2O)2CO
- Ethyl methyl carbonate, EMC, C4H8O3
- Additive:
- Vinylene carbonate, VC, (CHO)2CO
- Fluoroethylene carbonate, FEC, C3H3FO3
|
- Water content determination
- Karl Fischer (KF) Titration
- Hydrofluoric acid and chloride
- Density of solutions/solvents
- Conductivity
- Electrochemical measurements
- Color of electrolyte
|
KF Titrators
GT Titrators
DE/RE instruments
pH-meters
Conductivity meters
UV/Vis spectrophotometers |
| Separator |
Polypropylene, PP, or polyethylene, PE |
- Water content determination
- Karl Fischer (KF) Titration
|
KF Titrators |
| Cathode |
- Solvents (NMP: N-Methyl-2-Pyrrolidone)
- Lithium carbonate/hydroxide/chloride
- Lithium manganese oxide, LMO
- Lithium cobalt oxide, LCO
- Lithium nickel-cobalt-aluminum, NCA
- Lithium iron phosphate, LFP
|
- Water content determination
- Karl Fischer (KF) Titration
- Li2CO3, CO32−, LiOH, LiCl
- Co, Mn, Ni, Fe, Li, Al
- Al, As, Fe, Si, SiO42−
- GT and UV/Vis spectroscopy
- Fluoride, F−:
- General Titration (GT) and ISE
- Phosphate, PO43−:
|
KF Titrators
GT Titrators
DE/RE instruments
UV/Vis spectrophotometers
Ion-meters |
As mentioned previously, water content is a critical quality indicator and so must be determined across all four components. Since trace amounts of water are present in the materials, coulometric Karl Fischer titration is preferred.
If the liquid sample is soluble in the coulometric electrolyte, it is introduced directly into the titration cell using a syringe. All other insoluble samples are first heated in the KF furnace to evaporate the water and subsequently transferred to the titration cell. Other titration techniques, UV/Vis spectrophotometry, density measurement, pH, and conductivity determination also validate the battery components.
Common titration applications include determining impurities such as Cl−, assessing LiOH purity, and quantifying metal ion contents, including Co, Ni, Mn, and Fe.
1.4. Titration – Application Notes
Table 2 lists the application notes available for chemical analysis of the four main elements of lithium-ion batteries:
Table 2. Dedicated titration application notes available from AnaChem Applications Library – METTLER TOLEDO (mt.com). Source: Mettler-Toledo - Titration
| Analyte |
Battery element |
Application note |
| Water, H2O |
Anode, cathode, separator
Electrolytes, solvents |
Water Content of LIB Electrode Material Using Karl Fischer Titration (mt.com)
Water Content of Lithium-Ion Battery Electrolyte with KF Titration (mt.com)
Coulometric KF Titration of LIB Electrolyte (mt.com) |
Hydrofluoric acid,
HF
Lithium carbonate/
hydroxide
Lithium hydroxide,
LiOH |
Electrolyte
Cathode
Raw materials |
HF Content of Lithium-Ion Battery Electrolyte by Acid-Base Titration (mt.com)
Lithium Carbonate and Lithium Hydroxide Content of Cathode Material (mt.com)
Determining Purity of Lithium Hydroxide Using the InMotion Autosampler (mt.com)
Determine the Purity of Lithium Hydroxide by DispenSix Liquid Handler (mt.com) |
Nickel, manganese, cobalt
Cobalt |
Cathode
Cathode |
Total Metal in NMC Cathode of LIB with Titration Using DP5 Phototrode (mt.com)
Cobalt Content of Lithium Cobalt Oxide based Cathode Material (mt.com) |
Manganese
Cobalt Co,
nickel, Ni
Iron, Fe |
Cathode
Cathode
Cathode |
Manganese Content in NMC Cathode Material (mt.com)
Potentiometric Titration to Determine Cobalt and Nickel Content in LIB (mt.com)
Total Iron Content of Lithium Iron Phosphate Battery based Cathode Material (mt.com) |
Lithium, Li
Chloride, Cl− |
Electrolyte, raw materials
Cathode |
Potentiometric Determination of Li¯ ions in Batteries (mt.com)
Chloride Content of Lithium Cobalt Oxide based Cathode Material (mt.com) |
1.5. UV/Vis Spectrophotometry – Application Notes
The following table provides links to the currently available applications for UV/VIS Excellence and EasyPlus UV/VIS. They can be accessed through the AnaChem Applications Library-METTLER TOLEDO (mt.com).
Table 3. UV/Vis spectrophotometry application notes available from AnaChem Applications Library – METTLER TOLEDO (mt.com). Source: Mettler-Toledo - Titration
1.6. Density and Thermal Values – Application Notes
In addition to chemical analysis, selected physical parameters are determined to ensure quality. The links to currently available applications for density instruments can be accessed through the expertise library at the AnaChem Applications Library-METTLER TOLEDO (mt.com):
Table 4. Dedicated application notes for Physical Values determinations. The application notes are available from AnaChem Applications Library – METTLER TOLEDO (mt.com). Source: Mettler-Toledo - Titration
1.7. Case study – Chemical Analysis of Electrolyte
The following table presents a selection of relevant parameters that must be analyzed or determined in the electrolyte of a lithium-ion battery.
This list has been compiled based on data provided by multiple manufacturers and is a representative selection. The expected values are shown in the middle column, and the corresponding test methods are displayed in the right column. The green test methods can be conducted using METTLER TOLEDO analytical equipment:
Table 5. List of relevant parameters to be determined in the electrolyte of a Li-ion battery. The methods indicated in green show which analyses can be performed with METTLER TOLEDO instruments. Source: Mettler-Toledo - Titration
| Parameter |
Range |
Test method |
| Color |
< 50 |
APHA Pt/Co Hazen color scale |
| Water content |
< 15-20 ppm |
KF Coulometric Titration |
| Conductivity (25 °C) |
< 10.0 ± 0.5 mS/cm |
Conductivity meter |
| Free acid (HF) |
< 50 ppm |
Acid/base titration |
| Density (25 °C) |
1.20-1.25 g/cm3 |
Density meter |
Organic solvents /
LiPF6 / Additives |
Depending on
product w/w % |
Gas and ion chromatography |
| Sulfate |
< 10 ppm |
Nephelometry |
ev. Precipitation titration |
| Chloride |
< 1 ppm |
| Iron |
< 6 ppm |
AAS / ICP-OES |
ev. UV/VIS |
| Sodium |
< 10 ppm |
-- |
| Calcium |
< 10 ppm |
-- |
| Potassium |
< 10 ppm |
-- |
| Copper |
< 1 ppm |
-- |
| Chromium |
< 1 ppm |
-- |
| Nickel |
< 1 ppm |
-- |
| Lead |
< 5 ppm |
-- |
.

This information has been sourced, reviewed, and adapted from materials provided by Mettler-Toledo - Titration.
For more information on this source, please visit Mettler-Toledo - Titration.