Defined as a precious metal, silver has substantial value in the jewelry sector. Silver is too soft to be used directly, so it is often combined with other metals such as copper, nickel, zinc, cadmium, or palladium.
Sterling silver is a common alloy in jewelry, with 92.5 % pure silver and 7.5 % of an additional metal, such as copper or zinc, which increases durability and endurance against wear and tear.
Any item containing less than 90 % fine silver content is considered low standard. As a result, it is essential to have an accurate understanding of the composition of silver in alloys and fine jewelry products to ensure that all standard quality checks are met for all silverware.
Because of the importance of silver purity, various international standards, including EN ISO 11427, ISO 13756, GB/T 17823, GB/T 18996, and BIS 2113, govern the analysis of silver in silver jewelry and alloys.
This article details the potentiometric determination of silver content in silver coins and old silver jewelry samples using the DMi141-SC electrode on EasyPlus titrators (EasyCl/EasyPro) using sodium and potassium bromide as titrants.

Image Credit: Mettler-Toledo - Titration
Introduction
This article explains the procedure for the determination of silver content in silver jewelry using argentometric titration. The titration is measured with the DMi141-SC combined silver ring sensor and potassium bromide as the titrant.
Sample
- Silver Coin (Purity 999 %o / 99.9 %)
- Old Silver Jewelry (Purity ~ 933 %o / 93.3 %)
Sample size: 300 mg - 500 mg (0.3-0.5 g)
Preparation Procedure
- Prepare samples for factor analysis and sampling:
- Weigh 0.3 g of silver in a 100 mL glass titration beaker.
- Add five milliliters of 33 % HNO3 solution to the same beaker, cover with a watch glass, and gently warm until no nitrogen gas is produced.
- Allow it to cool, then rinse the watch glass in the beaker.
- To calculate the KBr standard solution factor
- Prepare a pure silver test sample and add 40 mL DI water.
- Titrate with 0.1 mol/L potassium bromide solution.
- Sample Titration
- To titrate a silver coin or jewelry sample, add 40 mL of DI water.
- Add a one percent aqueous solution of disodium dimethylglyoxime octahydrate to remove any palladium (if present). See the notes section for more information.
- Titrate it with 0.1 mol/L potassium bromide solution.
Chemistry
AgNO3 + KBr → AgCl + KNO3
Compound
Silver, M=107.86 g/mol; z=1
Chemicals:
- Deionized water (DI water)
- Disodium Dimethylglyoxime octahydrate (DMG), 1 %: Dissolve one gram in 100 mL water
- Potassium bromide, (KBr) 99 %
- 33 % Nitric acid: Take about 119.56 mL of concentrated nitric acid (69 %) in a 250 mL volumetric flask and make up the volume with DI water.
Titrant
- Potassium bromide, KBr, c(KBr) = 0.1 mol/L. Accurately weigh 11.901 g of KBr, transfer it to a 1000 mL volumetric flask, add 125 mL of DI water, swirl to dissolve, and make up the volume with DI water.
Instruments and Accessories
- Easy Cl (30060043)/Easy Pro (30060044)
- DMi141-SC (51109530), combined silver ring electrode.
- 100 mL Glass Titration beakers (00023517)
- Easy DirectTM titration software (30065449)
- S7 to BNC cable (30281915 - 1.2 m)
- XPR205 Analytical Balance (30355411)
- Hot plate
- Glass rod
- Watch glass
- 50 mL glass pipette.
Method
Parameters for factor determination:
Source: Mettler-Toledo - Titration
| . |
. |
| EQP /EP |
EQP |
| Relevant EQP |
1 |
| Titration type |
Direct |
| Sample ID |
Factor |
| Stir speed |
Maximum |
| Prestir duration |
45 seconds |
| Predispense |
0 mL |
| Control |
Normal |
| Sample size entry |
Variable weight |
| Calculation |
Consumption [mmol] |
| Multiple determination |
Yes |
| Report |
Long |
Parameters for sample determination:
Source: Mettler-Toledo - Titration
| . |
. |
| EQP /EP |
EQP |
| Relevant EQP |
1 |
| Titration type |
Direct |
| Sample ID |
Silver jewelry Spl |
| Prestir duration |
45 seconds |
| Predispense |
0 mL |
| Sample size entry |
Variable weight |
| Control |
User defined |
| dE (Titrant addition) |
9 mV |
| dVmin |
0.005 mL |
| dVmax |
0.6 mL |
| dE (value acquisition) |
0.5 mV |
| dt |
One second |
| tmin |
Three seconds |
| tmax |
30 seconds |
| Threshold |
100 mV/mL |
| Calculation |
Consumption [mmol] |
| Multiple determination |
Yes |
| Report |
Long |
Results
Factor Determination (n=5). Source: Mettler-Toledo - Titration
|
Silver Coin (mg/mL) |
Old Silver Jewelry (mg/mL) |
| R1 |
10.71 |
10.75 |
| R2 |
10.68 |
10.75 |
| R3 |
10.70 |
10.75 |
| R4 |
10.70 |
10.75 |
| R5 |
10.70 |
10.75 |
| STATISTICS |
|
|
| Mean |
10.70 |
10.75 |
| s |
0.004 |
0.002 |
| srel [%] |
0.010 |
0.020 |
Silver content in different silver samples (n=5). Source: Mettler-Toledo - Titration
|
Silver Coin (mg/mL) |
Old Silver Jewelry (mg/mL) |
| R1 |
988.04 |
930.19 |
| R2 |
992.83 |
931.27 |
| R3 |
993.53 |
930.47 |
| R4 |
995.24 |
931.09 |
| R5 |
995.98 |
931.12 |
| STATISTICS |
|
|
| Mean |
993.12 |
930.83 |
| s |
3.113 |
0.471 |
| srel [%] |
0.313 |
0.050 |
Titration Curve
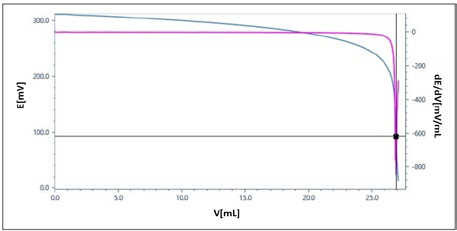
Representative titration curve for silver coin sample. Image Credit: Mettler-Toledo - Titration
Waste Disposal and Safety Measures
Prior to final disposal, neutralize the solution.
Remarks
- This application follows ISO ISO-11427-2014.pdf (iteh.ai) and GBT GB/T 17832-2008 English PDF (chinesestandard.net) standards.
- EasyPlus line titrators cannot adapt to the calculations indicated in ISO and GBT, hence they were conducted manually.
- To determine Factor (F): F = mAg/Vs mAg represents the mass of silver in milligrams.
- Vs represents the volume (mL) of KBr standard solution at EQP. For Sample Determination: mAg = F*Vs (VEQ). Where mAg is the mass of the test portion in mg.
- The mass of the test component in mg is calculated as wAg = mAg/ms * 103 ms.
- To prevent the AgCl precipitate from coagulating on the sensor, add five milliliters of five percent Triton X-100 to the sample solution. Alternatively, five milliliters of a 2.0 g/L PVA solution can be used.
- Proper sensor cleaning is essential to prevent dense precipitate accumulation, since greater RSD may occur.
- The amount of disodium dimethylglyoxime octahydrate depends on the palladium content. For every 100 milligrams of palladium, add 50 mL of this solution. If the DMG content is high, use a 250 mL glass titration beaker.

This information has been sourced, reviewed, and adapted from materials provided by Mettler-Toledo - Titration.
For more information on this source, please visit Mettler-Toledo - Titration.