Water impacts the quality of a wide range of products: chemicals, medicines, lithium-ion batteries, and food. Optimizing the water content present can help maintain product performance, shelf life and other important characteristics.
Karl Fischer (KF) titration is the most used analytical method for the determination of water content, because this method is highly selective to water.
There are two different KF titration techniques most often used, volumetric and coulometric, with the most appropriate based on the sample’s estimated water content.
Coulometric KF titration is usually chosen when measuring trace amounts of water in the parts per million (ppm) range. This method involves the electrochemical oxidation of iodine in a closed titration cell.
Volumetric KF titration leverages a precise burette and is better suited to the measurement of water content from approximately 100 ppm up to 100 %.
This article focuses on volumetric KF titration. While volumetric KF titration can be difficult, and the accuracy of results can be impacted by a range of factors. The use of automatic Karl Fischer titrators can help to address these challenges, potentially enhancing:
- Accuracy
- Repeatability
- Efficiency
- Ease of use
Careful attention to detail during measurement is essential to ensure accurate and reliable results.
This article covers several of these details, including the use of appropriate sample preparation to ensure accuracy and repeatability in water content determinations.

Image Credit: Mettler-Toledo - Titration
Sampling
Acquiring a Representative Sample
It is important to acquire a sample representative of the average amount of water in the entire sample.
- The solution should be shaken to distribute water in the liquid samples homogenously.
- It is possible to improve the release of water in solid samples by using a homogenizer that mostly eliminates the requirement for auxiliary reagents, for example, formamide for potato chips.
- Hygroscopic substances should be sampled rapidly using either a syringe or a dry spatula.
Appropriately Storing the Sample
Where necessary, samples should be stored in a tightly sealed bottle. Several things should be considered in sample storage, including:
- Glass bottles are preferred because these bottles are entirely gas tight. This means that atmospheric moisture is unable to penetrate the container walls.
- Bottles with small openings and septum stoppers should be used to minimize atmospheric moisture ingress.
- When working with liquid samples, the bottle should be rinsed two or three times with the sample to prevent contamination.

Image Credit: Mettler-Toledo - Titration
Sample Preparation
Selecting the Correct Sample Size and Ensuring Weighing Accuracy
Accurate weighing is the key to ensuring reliable results when preparing solutions for solid samples. This is also essential when back weighing syringes before and after KF titration.
It is possible to calculate sample size based on anticipated water content, but these calculations can be impacted by the titrant concentration. The table below shows recommended sample sizes.
Source: Mettler-Toledo - Titration
|
Titrant concentration |
| 5 mg/mL |
2 mg/mL |
1 mg/mL |
Expected water
content |
Sample size (g)
when a standard burette volume of 5 mL is used |
| 100 % |
0.015 |
- |
- |
| 60 % |
0.020 |
0.008 |
- |
| 40 % |
0.030 |
0.013 |
- |
| 30 % |
0.040 |
0.017 |
- |
| 10 % |
0.125 |
0.05 |
0.025 |
| 1 % |
1.25 |
0.5 |
0.25 |
| 0.1 % (1000 ppm) |
12.5 |
5 |
2.5 |
| 0.01 % (100 ppm) |
25 |
25 |
25 |
Checking the Sample’s pH
The Karl Fischer reagent is designed to function under specific pH conditions, generally between pH 5.5 and pH 8. If the pH is outside this range, the reaction between the sample and reagent may not proceed as anticipated.
The pH value can be adjusted to the ideal range by adding buffering agents, where the original sample is too acidic or basic.

Image Credit: Mettler-Toledo - Titration
Choosing the Ideal Preparation Techniques
Water should be completely released from the sample matrix to be available for reaction. Sample preparation is a key step towards achieving precise results.
Liquid sample preparation is straightforward, but it is essential to ensure sample solubility, for example, by achieving a complete release of water into the KF solvent.
Water molecules in solid samples are bound to the sample matrix in several different ways, meaning that an appropriate method must be employed to achieve efficient water release.
A number of techniques have been developed based on sample characteristics and the need to achieve full water release. These include:
- Direct titration
- External dissolution
- Gas-phase extraction
- External extraction
- Internal extraction
Source: Mettler-Toledo - Titration
| Sample Characteristic |
Sample Preparation Method and tips |
| Soluble, no side reaction |
Not specific: Direct titration |
| Insoluble, no side reaction, fast water release |
Internal extraction by longer stir time, homogenizer, improve solubility by auxiliary solvents |
| Insoluble, no side reaction, slow water release |
External extraction by long extraction time, suitable solvent, and large sample size |
| Soluble, non-homogeneous water distribution and low water content |
Direct titration with a suitable auxiliary solvent and a large sample size |
| Insoluble, side reactions with KF reagents and slow water release |
Gas-phase extraction with no reagent contact using a suitable heating time and temperature |

Image Credit: Mettler-Toledo - Titration
Liquid Sample Addition
The best sample addition method depends on the specific characteristics of the sample under investigation. It can be difficult to acquire a representative sample with certain samples. The table below features a range of tips on ensuring the sample is correctly transferred to the titration vessel.
Source: Mettler-Toledo - Titration
| Sample Characteristic |
Example |
Tips |
| High Water Content |
Perfumes, aqueous emulsions, alcoholic beverages |
- Use an appropriate volume of sample to avoid dilution errors and oversaturation of the KF reagent
|
| Low Water Content |
Olive oil, toluene, benzene, hexane |
- Increase the amount of sample used to ensure accurate measurement of water content
|
| Low viscosity |
Gasoline, diesel, acetic acid, hydrogen peroxide |
- Use a syringe with a thin needle attached to regulate the sample addition
|
| Viscous |
Syrup, glycerol, propylene glycol, transformer oil, lubricating oil |
- Predissolve the sample in a suitable solvent
- Use Visco-Spoon for easy handling
- Alternative: Use gas phase extraction
|
| Very viscous |
Molasses, ointments, creams, heavy fuel oil, epoxy resin |
- Pre-dissolve the sample in a suitable solvent
- Pre-heat the sample to lower the viscosity
|
| Hygroscopic |
Sodium bicarbonate, methanol, ethanol |
- Keep the sample in a tightly sealed container until use
- If possible, carry out the sample introduction in a dry room or glove box
|
Image Credit: Mettler-Toledo - Titration
Solid Sample Addition
Source: Mettler-Toledo - Titration
| Sample Characteristic |
Example |
Tips |
| Hard, Fatty |
Chocolate, solid fat |
- Grate the product and add the sample with a spatula
- Heat/ Melt the sample (e.g. chocolate)
|
| Soft, fatty, inhomogeneous |
Butter, margarine, edible fats |
- Homogenize the sample well: the water should be homogenously distributed
- Add the sample with a spatula; do not use a syringe because the water is released if it is pressurized
|
| Waxy |
Candle wax, paraffin, suppositories |
- Melt the sample at approx. 50 °C before filling it into a syringe, the syringe should be heated up together with the wax to prevent the sample from hardening during the weighing process
|
| Brittle Hard/soft pourable |
Salts, crystalline samples |
- Grind hard, coarse-grained samples in a closed, cooled analytical mill
- Add the sample with a weighing boat
|
| Finely powdered with very low water content |
Salicylic Acid, Talc Powder, Cellulose Powder, Magnesium Stearate |
- Either weigh the sample in a dry box or extract it externally
|
| Soft, Jelly-like |
Jellied fruits, jelly bears, and almond paste |
- Cut the sample into small pieces with scissors or a knife, and add the sample with a spatula.
|
| Insoluble solid |
Polymer pellets, plastic chips |
- Use a gas phase extraction
|

Image Credit: Mettler-Toledo - Titration
The Karl Fischer Titrator
Condition Before Use
Equipment should be conditioned before titration for two key reasons:
- Conditioning equipment eliminates any water that may have been introduced into the titration system, preventing inaccurate water content measurement.
- Conditioning removes residual contaminants potentially present on the equipment. For example, a syringe should be rinsed once or twice with the sample to be titrated before analysis.
Tightening Connections
All connections should be tightened prior to beginning the titration to help prevent excessive disruption from atmospheric moisture. In the case of volumetric titration, it is also essential to ensure that all connections on the KF cell insert are tightly secured. Sealing rings should be used wherever air can enter the system, for example, between all bottles, connectors, and dry tubes with molecular sieves.

Image Credit: Mettler-Toledo - Titration
Regularly Calibrating the Titrator
If titrators are not regularly calibrated, inaccuracies may go unnoticed, potentially leading to errors in titration results. For example, failing to properly calibrate a burette used to measure the Karl Fischer reagent may result in an incorrect amount of reagent being added.
It is advisable to use a calibration service to avoid any mistakes during the sensitive KF titration process.
Routinely Performing a System Suitability Test
The system suitability test is essentially the final test of operating qualification, demonstrating the correct and error-free operation of the titration system. This test should be regularly performed to ensure accuracy.
General system suitability tests (GSSTs) are performed in line with standard operating procedures. These tests encompass:
- Purpose and scope
- Acceptance criteria
- Necessary chemicals and materials
- Step-by-step procedure
- Results presentation

Image Credit: Mettler-Toledo - Titration
Reagents
Using Fresh Karl Fischer Reagents
Fresh KF reagents should be used in all cases to ensure they have the correct concentration and are free of impurities.
KF reagents can degrade over time due to exposure to contaminants, including moisture and air.
In some instances where solvents are not fresh, but it is still necessary to acquire a measurement, the required conditioning time may be extended.
Regularly Determining Titrant Concentration
It is important to determine the concentration of titrants to accurately determine a substance’s concentration. It is important to note that fresh titrants created using KF reagents typically have a concentration that is approximately 10 % higher than the anticipated concentration.
A daily concentration determination is typically recommended, but users based in countries where the temperature varies considerably during the day benefit from determining the concentration of titrant every two to four hours.

Image Credit: Mettler-Toledo - Titration
Using Auxiliary Solvent (Depending on Sample)
Certain sample types may not be fully dissolvable by the solvent. This issue can be avoided by adding co-solvents to enhance sample solubility in the titration cell.
Commonly used co-solvents include:
- Chloroform
- Toluene
- Formamide
Image Credit: Mettler-Toledo - Titration
Using Safer Reagents
New advances in reagents have led to the development of safer options formulated without imidazole, which is a carcinogenic, mutagenic, and reprotoxic (CMR) substance. The use of these safer reagents can help ensure operator safety.
Avoiding Side Reactions
Side reactions in volumetric KF titration occur when other substances in the sample react with the reagents. Side reactions should be avoided because they can adversely affect water content measurement accuracy.
Selecting the Right Reagent
Choosing the most appropriate reagent for a specific sample ensures the highest accuracy based on application and sample requirements. The table below shows the advantages and disadvantages of various reagent types.
Source: Mettler-Toledo - Titration
| Reagents |
+ |
– |
| One-component |
Simple handling,
favorably priced |
Titer is less stable,
titration speed slower |
| Two-component |
High titration speed,
stable titer |
Solvent capacity
restricted |
Ensuring the Ideal Data Transfer
SmartChemicals reagents transfer identifying data to the titrator via a contactless SmartReader, eliminating risks of errors typically associated with manual transcription. SmartReaders on select titrators enable the instant transfer of:
- Reagent name
- Lot and batch number
- Concentration
- Shelf life, open date, and expiration
This straightforward information transfer improves analysis speed and accuracy.
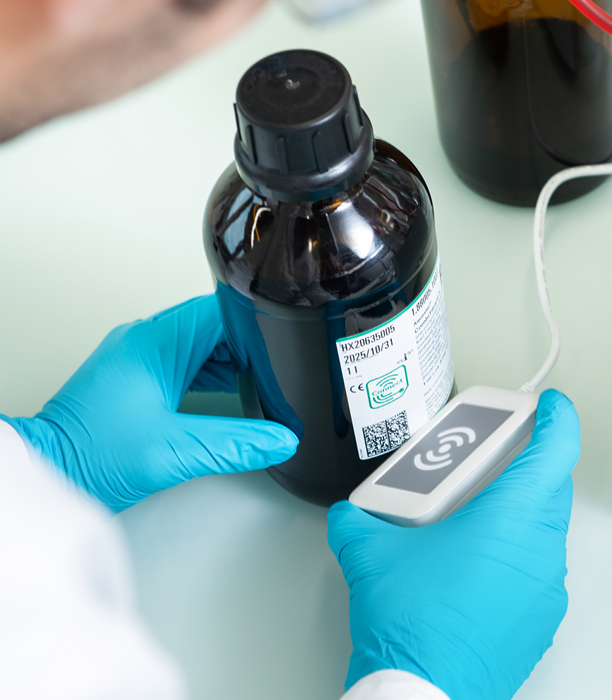
Image Credit: Mettler-Toledo - Titration
Performing a KF Titration
Assuring Low Drift Prior to Commencing Titration
Drift indicates the amount of water entering the titration stand over a defined period. This is achieved through the titration of dry solvent for a defined amount of time.
Implementing a number of small steps can help assure a low drift value, including:
- Titrating in suitable workspaces with the right temperature, low humidity, and no strong ventilation
- Checking system seals and tightness
- Routinely renewing and drying exhausted molecular sieves
- Gently shaking the KF titration cell to collect traces of water from the glass walls.
Performing a Blank Titration
Blank titration allows the baseline moisture content in the reagents and equipment to be determined. This can then be subtracted from the sample titration’s moisture content. This approach is widely used in conjunction with an external extraction or dissolution technique.
Blank value determinations are executed differently depending on the technique used to extract the sample, but the basic steps always include ensuring the sample is not present and running a titration using just the KF reagents and solvents used in the titration process.

Image Credit: Mettler-Toledo - Titration
Results and Data Management
Ensuring Reliable High-Quality Data
Reliable data management is key to trustworthy results. The LabX® laboratory software is designed to optimize workflows from sample data entry through to measurement, reporting, and export.
LabX® allows its users to easily:
- Simplify daily routines using preprogrammed SOPs
- Eliminate manual data entry to prevent transcription errors
- Improve efficiency via the automatic data capture and real-time storage of all data
- Centralize and automate results documentation.
LabX® also fully supports current regulatory requirements through the inclusion of key security features, including:
- Electronic signatures
- Audit trails
- User rights

Image Credit: Mettler-Toledo - Titration
The Ideal Titrator for Volumetric KF Titration

EVA V1 KF Titrator. Image Credit: Mettler-Toledo - Titration

EVA V3 KF Titrator. Image Credit: Mettler-Toledo - Titration
The EVA Volumetric Karl Fischer Titrator is designed to improve volumetric KF titration workflows, maximizing productivity via an intuitive OneClick user interface, FFA control technology, and plug-and-measure accessories.
The EVA can efficiently determine water content in a diverse array of sample matrices, with its innovative control system ensuring rapid and reliable results every time by accelerating the KF titration process while simultaneously improving accuracy.
The EVA Volumetric Karl Fischer Titrator also features:
- Support for challenging samples, with a range of easy-to-use accessories available to accommodate a wide range of applications.
- Comprehensive user management, with full control over access rights and permissions available as standard with every modem.
- A flexible, modular design makes it easy to exchange components and configure the system to accommodate different application requirements.
- Effortlessly balanced integration, ensuring accurate automatic weight transfer while eliminating errors and delays via straightforward connection and communication.
Acknowledgments
Produced from materials originally authored by Mettler Toledo.

This information has been sourced, reviewed, and adapted from materials provided by Mettler-Toledo - Titration.
For more information on this source, please visit Mettler-Toledo - Titration.