Analyzing microplastics (MPs) in real environmental matrices requires the unambiguous identification of particles to distinguish them from particles of biogenic origin, such as particulate organic matter, and particles belonging to the microlitter in general, such as plastic additives or non-plastic artificial and natural fibers.1-7
The standard non-invasive techniques used to quantify suspected MPs are currently optical microscopy using dye staining and electron and fluorescence microscopy, since the unambiguous identification of polymers is not provided.8-10
Conversely, vibrational spectroscopy using Fourier Transform infrared (FTIR) and Raman spectrometers is a widely recognized non-destructive technique that enables the characterization of a range of particles, including polymers that cannot be detected in the aforementioned techniques.
After the optical investigation, some particles will be selected for further analysis, which is typically performed using an FTIR spectrometer. Overestimation and underestimation of MPs can occur, so it is advisable to analyze larger particles or fibers (>500 μm) using attenuated total reflection - FTIR (ATR - FTIR).
To ensure unequivocal identification, particle quantification (microscopic counts) and subsequent analysis of selected particles are particularly time-consuming. Aside from this drawback, selecting just a few particles may not fully represent what is actually present in the sample.
Punctual analysis of particles using techniques such as micro-FTIR and micro-Raman can be equally time-consuming. However, particle analysis via micro-FTIR enables the unequivocal identification of MPs and other microliter components, particularly small microplastics (SMPs) below 100 µm. These are also reliably quantified by microscopic count, avoiding over- or underestimation.3,4,6,11,12
Particle analysis can be performed using the Particles WIZARD section of the Thermo Scientific™ OMNIC™ Picta™ Software, the instrument software of the Thermo Scientific™ Nicolet™ iN10 MX Infrared Imaging Microscope. This analysis can be performed on any filter suitable for microplastic analysis, including silicon oxide filters and aluminum oxide filters.
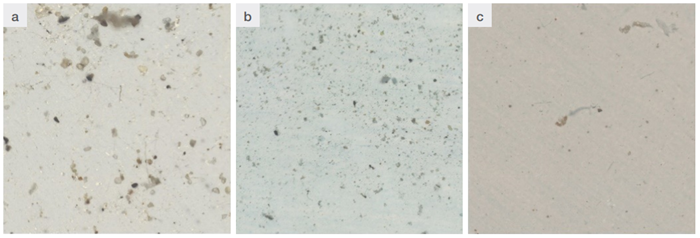
Figure 1. Examples of mosaic or count field: a) in permafrost sample, b) in soil sample, c) in seawater. Image Credit: Thermo Fisher Scientific - Vibrational Spectroscopy
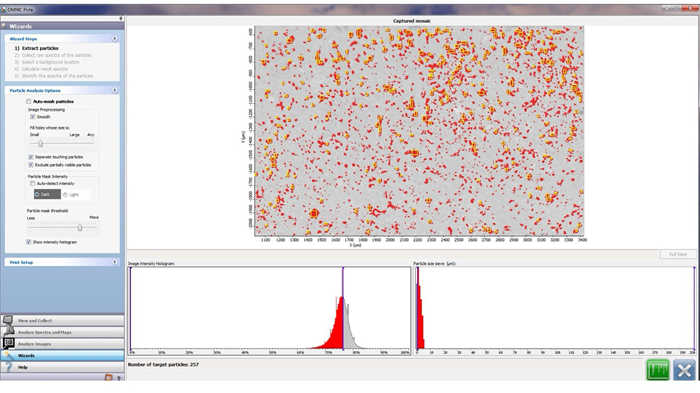
Figure 2. Particle Analysis via WIZARD: Example of how to select the particles on the count field. Image Credit: Thermo Fisher Scientific - Vibrational Spectroscopy
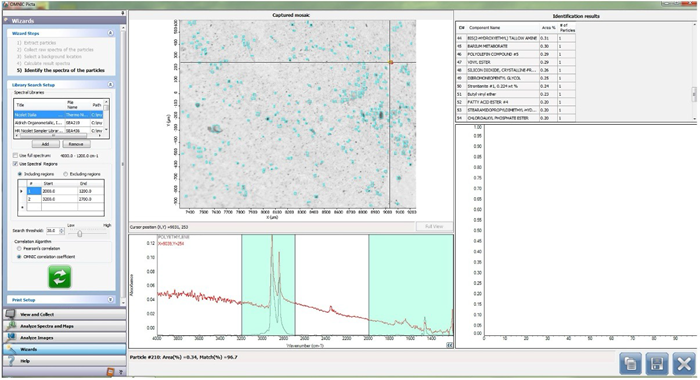
Figure 3. Particle Analysis via WIZARD: The spectra of the particles are identified. Image Credit: Thermo Fisher Scientific - Vibrational Spectroscopy
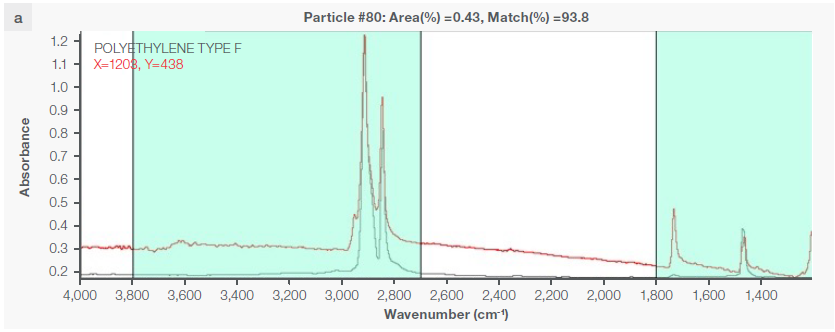
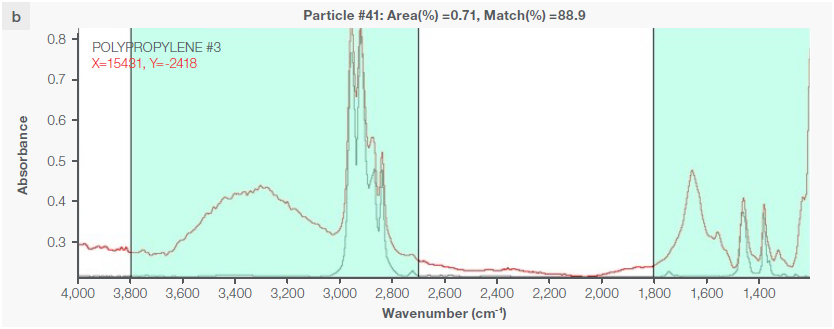
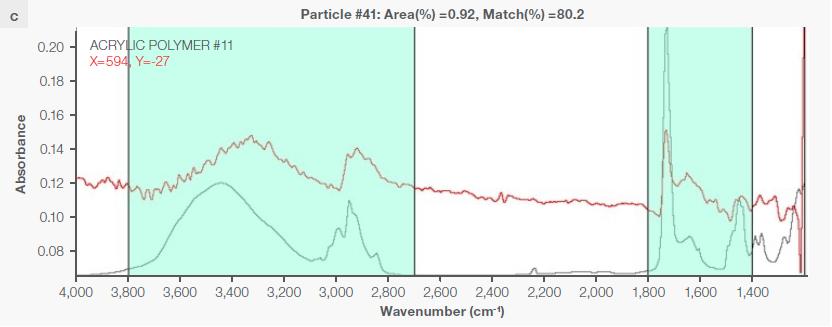
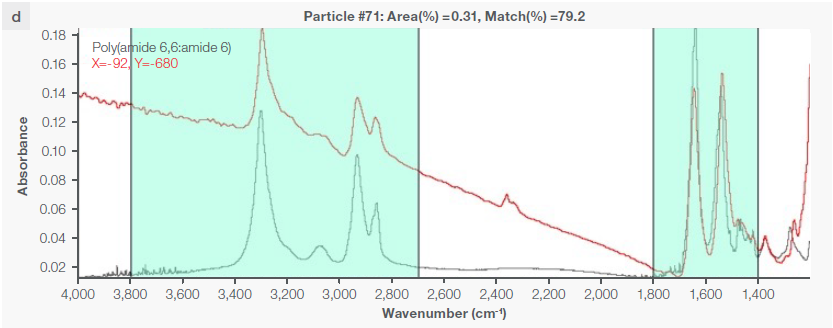
Figure 4. Some exemplary spectra of polymers optimally identified with a match percentage > 80 %: a) polyethylene, b) polypropylene, c) acrylic, d) polyamide 6. Image Credit: Thermo Fisher Scientific - Vibrational Spectroscopy
Experimental Considerations
Particle Selection
An area is framed using the micro-FTIR’s objective with a spatial resolution of 100 µm. A mosaic of definite dimensions is then drawn: In environmental samples, these dimensions will be 2000 µm x 1400 µm.
This mosaic becomes the ‘count field’ or ‘count area’ (Figure 1). Once the mosaic is saved, the Particles Analysis function can start, accessed via the WIZARD section of OMNIC™ Picta™ software.
This function detects any particles present on the filter’s surface in relation to the brightness value in the specific count area. In this context, brightness value is defined as each particle’s brightness ratio in contrast to the background (Figure 2).
When using the software, the user should first uncheck the Auto-mask particles option before selecting ‘Smooth’, ‘Separate touching particles’, and ‘Exclude partially visible particles’ in the Image preprocessing section. The latter two are especially important for microscopic counting.
Next, in Particle Mask intensity, ‘Show intensity histogram’ should be selected after unchecking ‘Auto-detect intensity’. The intensity histogram allows particles to be selected for analysis later.
Particles are enclosed in rectangles called bounding boxes. The image intensity histogram is required to select an adequate number of particles because the amount of microplastics present in that field is not known a priori.
Spectral and background interferences can affect the brightness ratio, causing the software to detect fewer particles. Therefore, the Particle size sieve function is used to diminish any potential interference signal (Figure 2).
After detection, the particles' raw spectra are collected. Following this collection, a background location is selected on the count field. This combination of raw spectra and background spectra enables calculation of the resulting particle spectra.
The final step is the comparison of the resulting spectra to reference libraries, with a resulting match percentage identifying each spectrum of each particle (Figure 3). The coordinates of each particle in the count field are also retrieved, allowing each particle to be unequivocally identified.
Instrumental characteristics reveal that the optimal range of match percentage is ≥ 65 %, but it is possible for the match percentage to be > 80 % (Figure 4), depending on the specific pretreatment used.
It is important to eliminate spectral and background interferences during pretreatment, especially when a purification procedure is applied during the filtration.3,4 This approach ensures the efficiency of particle selection, improving the identification match percentage of each particle over the optimal range.
It is impossible to optimally identify and count an identification match percentage of < 65 %, meaning that MPs’ abundance will be underestimated.
Microscopic Counting for Microplastics and Microlitter
Microscopic counting has been used with phytoplankton, pollen, bacteria, spores, and MPs.3,4,6,13-22 A key benefit of microscopic counting is its capacity to eliminate doubt surrounding the number of cells, organisms, or particles present within valid computable limits and degrees of chance.
Round or square filters can be easily employed to support the counting process. Analyzed filter areas, such as count fields or count areas, must sufficiently represent the entire filter to avoid issues regarding representativeness and reproducibility.
Figure 5 shows how representative measurement areas of the same size can be chosen on the surface of the filter. The approach used in the Bürker chamber can also be applied to square filters. When analyzing MPs, the number of analyzed filtering areas must be greater than or equal to 20 to acquire robust and meaningful quantification.
The loading of the filters cannot be known in advance, so count areas with different abundances should be considered to avoid issues regarding the accuracy of the extrapolation of microplastics, cells, organisms, or bacteria findings. As a result, a randomized approach without overlapping (Figure 5d 3-5,7) is the most suitable.
The number of particles analyzed should be at least 4000 so that the count can be considered reliable and representative. The microscopic count is only considered robust when these two conditions are met, with the quantification sure to be unencumbered by under- or over-estimation.
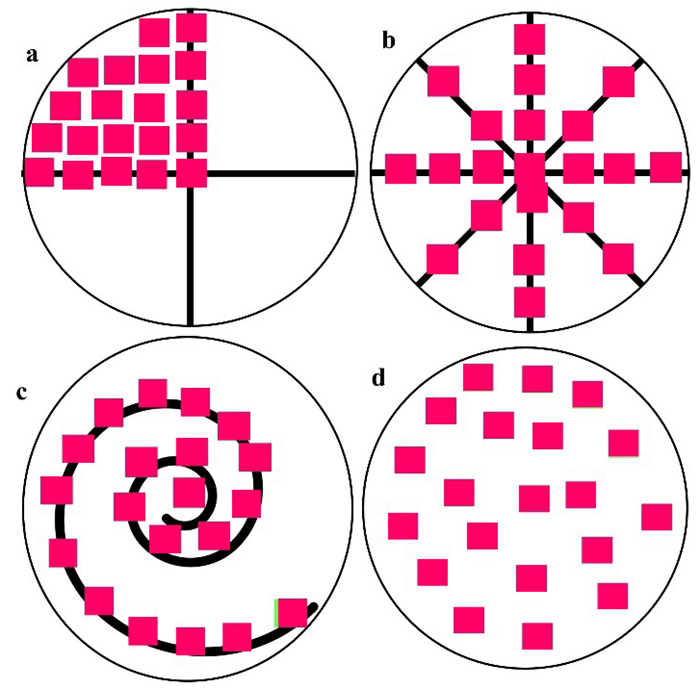
Figure 5. Different approaches may be used for representative measurement areas on filters; at least 20 count areas or count fields should be considered. These approaches can be employed on filters of different diameters and materials (e.g., aluminum oxide, silicon oxide, PTFE). Example a) represents a quarter of the filters; b) represents the cross-section of the four axes c) represents a helical assembly; and d) represents a randomized assembly. Image Credit: Thermo Fisher Scientific - Vibrational Spectroscopy
It is necessary to multiply the total counts from each filter by the appropriate microscope conversion (optical factor F) and volume or dilution factors3-5,7 to obtain the absolute abundance, measured, for example, as the number of MPs per kg, number of MPs per L, or number of MPs per m.
Equations for calculating the abundances are defined as:3-5,7
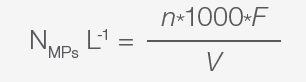 |
Equation 1. |
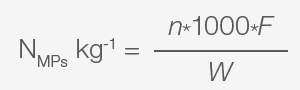 |
Equation 2. |
In these equations:
- NMPs L-1 or NMPs kg-1 are the total abundance in the samples analyzed
- V is the volume of water analyzed
- W is the weight of soil, sediments, etc., analyzed
- n is the sum of all the plastic particles in the count fields analyzed
- F is the optical factor that is calculated according to the equation below
 |
Equation 3. |
Density, shape, and size are all essential particle and fiber characteristics that impact their permanence and transport in the environment, as well as their inhalation or ingestion by humans and other animals.
Like aerosol particles, microplastic particles and fibers may feature a range of irregular shapes, which can be described by shape descriptors.23-26
Particle Selection and Counting Using WIZARD
The iN10’s OMNIC™ Picta™ software’s Particles Analysis function allows particles to be identified and counted, and the length and width of each particle are also retrieved as part of the analysis.
Selecting particles in the software (Figure 1) encloses them in rectangles called bounding boxes, so named because they correspond to the smallest rectangles that enclose each particle’s shape. It is then possible to categorize particles’ shapes based on the aspect ratio of the bounding boxes.
Calculation of MPs’ Aspect Ratio and Volume
Aspect ratio (AR) is defined as the ratio between the maximum length (L) and the maximum width (W) of the bounding box that encloses the shape in question.
For example, particles are considered spherical when the AR values are ≤1, ellipsoidal when AR ≥ 2, and cylindrical when AR ≥ 3. AR enables the calculation of particle and fiber volumes according to their geometrical shape: sphere, ellipse, or cylinder. Since the particles have been optimally identified, it is also possible to retrieve their density, allowing the calculation of each particle’s weight.
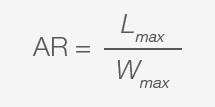 |
Equation 4. |
Conclusions
Successful MP analysis requires users to discern polymers from the rest of the microlitter components and any other particles present in the environmental matrix.
It would be extremely time-consuming to analyze hundreds of particles individually to ensure robust quantification, potentially taking several days to analyze every FTIR spectra retrieved.
In addition, performing a count of MPs separately from analysis via vibrational spectroscopy would contribute significantly to the time required for analysis, potentially adding as much time as performing a complete analysis of the entire filter.
Instead, particle analysis software allows a single particle to be unambiguously located by its spatial coordinates in each field count, with its spectrum, width, and length collected and optimally identified.
The Particles Analysis mode allows quantification by microscopic count to be performed simultaneously alongside spectral identification. Each count field can be saved with the filename extension .map, enabling easy analysis of each particle in a precise count field to confirm or verify spectral identification.
Particle analysis software makes microplastics analysis significantly less time-consuming and considerably more robust.
References and Further Reading
- Löder, M.G.J. and Gerdts, G. (2015). Methodology Used for the Detection and Identification of Microplastics - A Critical Appraisal. Marine Anthropogenic Litter, (online) pp.201–227. https://doi.org/10.1007/978-3-319-16510-3_8.
- Wirnkor, V.A., Enyoh Christian Ebere and Ngozi, V.E. (2019). Microplastics, an emerging concern: A review of analytical techniques for detecting and quantifying... Analytical Methods in Environmental Chemistry Journal. (online) https://doi.org/10.24200/amecj.
- Corami, F., et al. (2020). A novel method for purification, quantitative analysis and characterization of microplastic fibers using Micro-FTIR. Chemosphere, 238, p.124564. https://doi.org/10.1016/j.chemosphere.2019.124564.
- Corami, F., et al. (2021). Small microplastics (<100 μm), plasticizers and additives in seawater and sediments: Oleo-extraction, purification, quantification, and polymer characterization using Micro-FTIR. Science of The Total Environment, 797, p.148937. https://doi.org/10.1016/j.scitotenv.2021.148937.
- Corami, F., et al. (2022). Occurrence and Characterization of Small Microplastics (<100 μm), Additives, and Plasticizers in Larvae of Simuliidae. Toxics, 10(7), p.383. https://doi.org/10.3390/toxics10070383.
- Ivleva, N.P. (2021). Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chemical Reviews, 121(19), pp.11886–11936. https://doi.org/10.1021/acs.chemrev.1c00178.
- Rosso, B., et al. (2022). Quantification and characterization of additives, plasticizers, and small microplastics (5–100 μm) in highway stormwater runoff. Journal of Environmental Management, 324, p.116348. https://doi.org/10.1016/j.jenvman.2022.116348.
- Möller, J.N., Löder, M.G.J. and Laforsch, C. (2020). Finding Microplastics in Soils: A Review of Analytical Methods. Environmental Science & Technology, 54(4), pp.2078–2090. https://doi.org/10.1021/acs.est.9b04618.
- Girão, A. V. (2022). SEM/EDS and optical microscopy analysis of microplastics. In Handbook of Microplastics in the Environment (pp. 57-78). Cham: Springer International Publishing.
- Bai, R., et al. (2023). Microplastics are overestimated due to poor quality control of reagents. Journal of Hazardous Materials, (online) 459, p.132068. https://doi.org/10.1016/j.jhazmat.2023.132068.
- Mehdinia, A., et al. (2020). Identification of microplastics in the sediments of southern coasts of the Caspian Sea, north of Iran. Environmental Pollution, 258, p.113738. https://doi.org/10.1016/j.envpol.2019.113738.
- Vianello, A., et al. (2013). Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuarine, Coastal and Shelf Science, 130, pp.54–61. https://doi.org/10.1016/j.ecss.2013.03.022.
- Algal Culturing Techniques. (2004). (online) Elsevier. Available at: https://shop.elsevier.com/books/algal-culturing-techniques/andersen/978-0-12-088426-1.
- Brierley, B., et al. Guidance on the quantitative analysis of phytoplankton in Freshwater Samples. (online) Available at: https://nora.nerc.ac.uk/id/eprint/5654/1/Phytoplankton_Counting_Guidance_v1_2007_12_05.pdf.
- Comtois, P., Alcazar, P. and Néron, D. (1999). Aerobiologia, 15(1), pp.19–28. https://doi.org/10.1023/a:1007501017470.
- Gough, H.L. and Stahl, D.A. (2003). Optimization of direct cell counting in sediment. Journal of Microbiological Methods, 52(1), pp.39–46. https://doi.org/10.1016/s0167-7012(02)00135-5.
- Huppertsberg, S. and Knepper, T.P. (2018). Instrumental analysis of microplastics - benefits and challenges. Analytical and Bioanalytical Chemistry, 410(25), pp.6343–6352. https://doi.org/10.1007/s00216-018-1210-8.
- Lisle, J.T., et al. (2004). Comparison of Fluorescence Microscopy and Solid-Phase Cytometry Methods for Counting Bacteria in Water. Applied and Environmental Microbiology, 70(9), pp.5343–5348. https://doi.org/10.1128/aem.70.9.5343-5348.2004.
- Mazziotti, C., Fiocca, A. and M.R. Vadrucci (2013). Phytoplankton in transitional waters: Sedimentation and counting methods. Transitional Waters Bulletin, (online) 7(2), pp.90–99. https://doi.org/10.1285/i1825229Xv7n2p90.
- Muthukrishnan, T., et al. (2017). Evaluating the Reliability of Counting Bacteria Using Epifluorescence Microscopy. Journal of Marine Science and Engineering, 5(1), p.4. https://doi.org/10.3390/jmse5010004.
- Oßmann, B.E., et al. (2018). Small-sized microplastics and pigmented particles in bottled mineral water. Water Research, (online) 141, pp.307–316. https://doi.org/10.1016/j.watres.2018.05.027.
- Tong, H., et al. (2020). Occurrence and identification of microplastics in tap water from China. Chemosphere, (online)252, p.126493. https://doi.org/10.1016/j.chemosphere.2020.126493.
- Adachi, K. and Buseck, P.R. (2014). Changes in shape and composition of sea-salt particles upon aging in an urban atmosphere. Atmospheric Environment, (online) 100, pp.1–9. https://doi.org/10.1016/j.atmosenv.2014.10.036.
- Bharti, S.K., et al. (2017). Characterization and morphological analysis of individual aerosol of PM10 in urban area of Lucknow, India. Micron, 103, pp.90–98. https://doi.org/10.1016/j.micron.2017.09.004.
- Chen, L., et al. (2007). Emissions from Laboratory Combustion of Wildland Fuels: Emission Factors and Source Profiles. 41(12), pp.4317–4325. https://doi.org/10.1021/es062364i.
- Hamacher-Barth, E., Jansson, K. and Leck, C. (2013). A method for sizing submicrometer particles in air collected on formvar films and imaged by scanning electron microscope. (online) https://doi.org/10.5194/amtd-6-5401-2013.
Acknowledgments
Produced from materials originally authored by Fabiana Corami and Beatrice Rosso from the Institute of Polar Sciences, CNR ISP; and Barbara Bravo from Thermo Fisher Scientific.

This information has been sourced, reviewed and adapted from materials provided by Thermo Fisher Scientific - Vibrational Spectroscopy.
For more information on this source, please visit Thermo Fisher Scientific - Vibrational Spectroscopy.