Polyolefins, specifically polypropylene and polyethylene, are high-performance plastics obtained from propylene and ethylene. These materials are crucial in many different sectors because of their strength, flexibility, and excellent chemical resistance.
They are widely used in packaging solutions such as bags, containers, and films, simultaneously providing convenience and protection. In the construction field, polyolefins play a vital role in products such as insulation materials, roofing membranes, and piping systems because of their long service life and resilience.
In the automotive industry, polyolefins are preferred for their endurance to impact, low density, and ability to combat severe environmental factors, making them ideal materials for components requiring both strength and efficiency.
As sustainability begins to be prioritized, innovations in polymer processing and the development of biodegradable and recycled-content polyolefins are advancing the material solutions of tomorrow. These developments help manufacturers and consumers simultaneously achieve their performance goals and environmental standards.
In 2024, the polyolefin market volume stood at $231 million: its expected growth rate of 5.29 % means it is forecast to reach $372 million by 2034.
The Asia Pacific region dominates this market volume with a share exceeding 50 % while North America and Europe make up most of the balance of production.
Polyolefin Catalysis
The efficient production of specific polyolefins requires the development and selection of fitting catalysts. Many chemical companies are increasingly focusing on catalyst development, which involves substantial investment for catalysis screening in research facilities.
Catalysis screening is performed on a laboratory scale, using batch reactors, where the polymerization runs are very short, taking only one hour. Typically, there will be multiple batch reactors in a single laboratory, helping experimental data be collected as rapidly as possible.
One important feature of using batch reactions for polyolefins, which is different from the continuous processes that are used in plant production, is that steady-state conditions (in terms of ratios of key constituents) are impossible to attain without on-line process gas analysis.
For example, in the case of polyethylene (PE), hydrogen is used to terminate the polymer chains. When the reaction commences with a specific H2/C2H2 concentration ratio, the H2/C2H4 ratio rapidly becomes depleted if no more H2 is added to the system. This means that the polymers produced at the beginning of the run will have a much lower average molecular weight than those produced at the end.
The problem is illustrated in Figure 1. It becomes impossible to develop catalysts to run under static conditions, with the aim of achieving a specific polymer product. The solution is to use on-line analysis combined with system-controlled metering of H2, which is introduced using a mass flow controller.
This solution has been implemented in some situations and experimental settings where constant H2/C2H4 ratios have been maintained. This results in the achievement of a narrow and symmetrical distribution of polymer molecular weights. In this way, catalyst screening is being executed in a way that mimics actual steady-state plant conditions, therefore generating statistically valid data for catalyst performance.
A similar effect can be seen with α-olefins such as 1-butene and 1-hexene, which are used in PE polymerization experiments to produce the required polymer grades. During production, these key components are depleted, but they can be regulated at a consistent ratio proportional to C2H4 by controlled introduction based on online analysis.
Analogous regulation schemes (based on H2/C3H6 and 1-butene/C3H6 ratios) are used for polypropylene (PP) experiments. Similarly, C2H4/C3H6 can be regulated for ethylene-propylene co-polymer production.
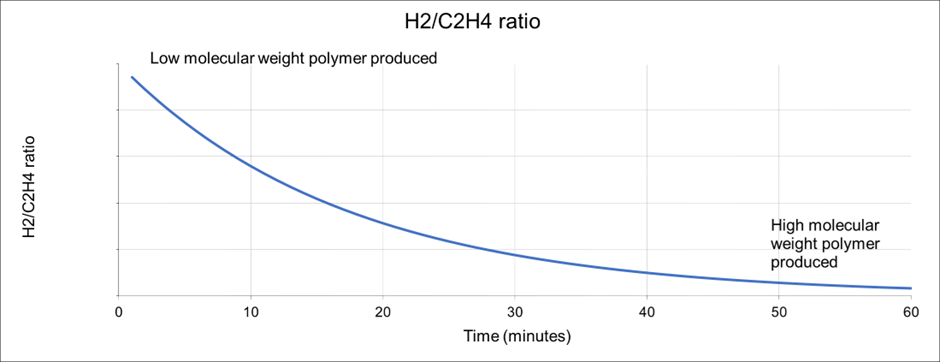
Figure 1. Hydrogen depletion and the effect on polymer molecular weight. Image Credit: Thermo Fisher Scientific – Environmental and Process Monitoring Instruments
HDPE Process Data
The key objective of the aforementioned research is to obtain a polymer with a very tight (heavy) molecular weight distribution, while achieving minimum hydrogen consumption. The experiments test different catalysts to fulfil this. In this case, research reactors are currently working with one factor of 10 less hydrogen than the full-scale plant.
Replicating the use of this new catalyst in production reactors will notably reduce hydrogen consumption whilst producing a noticeably higher-density polymer product. This can be verified by using mass spectrometry (MS). Figure 2 shows the ability of MS to measure gas species at the catalyst, even at very low concentrations: the concentration of H2 is ~500 parts per million (ppm), while the concentrations of hexene-1 and hexene-2 are ~100 ppm or less.
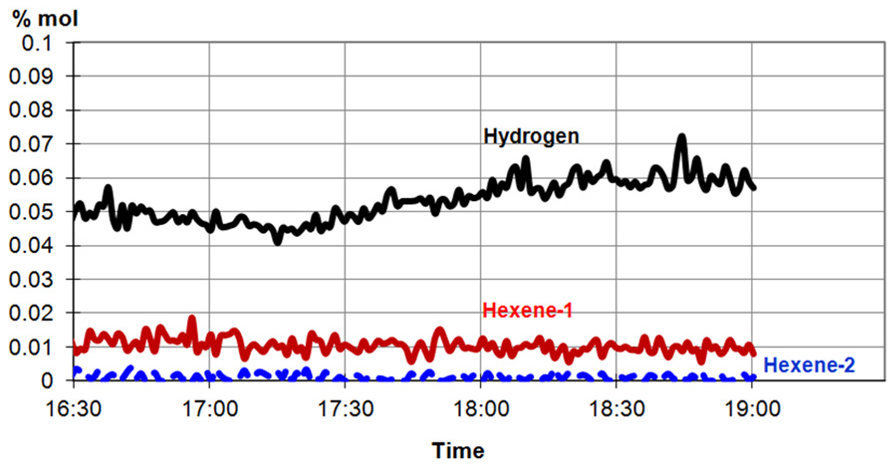
Figure 2. MS analysis of very low concentrations of H2, hexene-1, and hexene-2. Image Credit: Thermo Fisher Scientific – Environmental and Process Monitoring Instruments
Analyzer Selection
A major polyolefin producer used gas chromatography (GC) to perform on-line process gas analysis. The requirement for multiple reactors and the shortest possible cycle times necessitated dedicated GC units for each reactor. However, even in this case, the fastest possible cycle time was five minutes for analysis up to C-4 (or eight minutes up to C-6), and regulation was poor. Therefore, MS was considered, as this is known to be an efficient technique, usually providing 20 times faster analysis compared to GC.
After exhaustive evaluation of two types of MS, the Thermo Scientific™ Prima™ series Process Mass Spectrometer was chosen. The Prima spectrometer is a highly robust, precise, and reliable process gas analyzer; there are over 1500 units installed worldwide in chemical plants, pharmaceutical processes, iron and steel works, and in research laboratories. Before this specific spectrometer was selected, it was evaluated in a comparative trial, where a quadrupole process MS, a process GC, and a laboratory GC were also used to evaluate a selection of samples.
The laboratory GC proved it could achieve very high accuracy when tested with a variety of certified gas mixture blends. The results shown in Figure 3 indicate that the results obtained from the Prima series process mass spectrometer were highly similar to the laboratory GC.
It can be seen that the Prima series process MS produced more accurate data than the process GC. However, it is evident that not every type of mass spectrometer is suitable for this type of analysis.
The quadrupole MS (QMS) was shown to give poor accuracy for this application, due to the unstable, poor response for H2 exhibited by quadrupole systems because of the low energy of the ion beam, typically 10 eV or less. The Prima series process MS, however, uses a very high ion energy (1000 eV), resulting in very high accuracy and reproducibility.
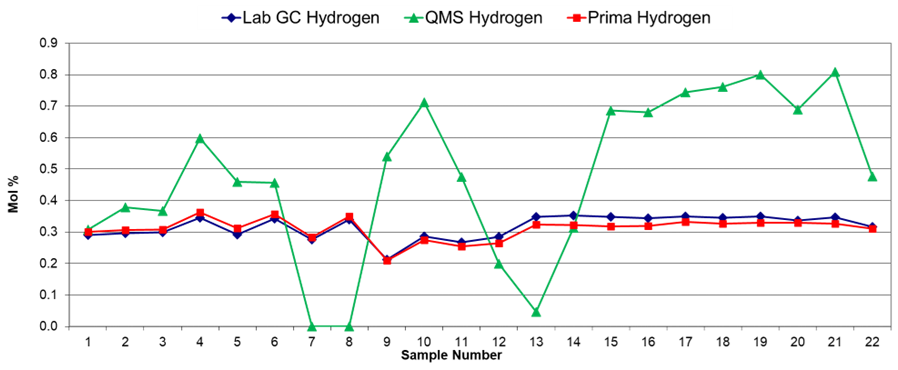
Figure 3. Hydrogen data from lab GC, quadrupole MS and Prima series process MS systems. Image Credit: Thermo Fisher Scientific – Environmental and Process Monitoring Instruments
Comparison can also be made between the Prima series process MS and process GC when the subject molecule is ethylene. Figure 4 gives a data set of 55 analysis points from the lab GC and both the MS and process GC. For consistency, the MS only samples at the same rate as the GCs, but MS can provide data at a much faster rate if needed.
It can be seen that there is generally good agreement between the three analyzers, with the closest alignment occurring between the lab GC and MS. The outlier is the process GC, where there are several instances of data that do not align with the results obtained from the two other analyzers.
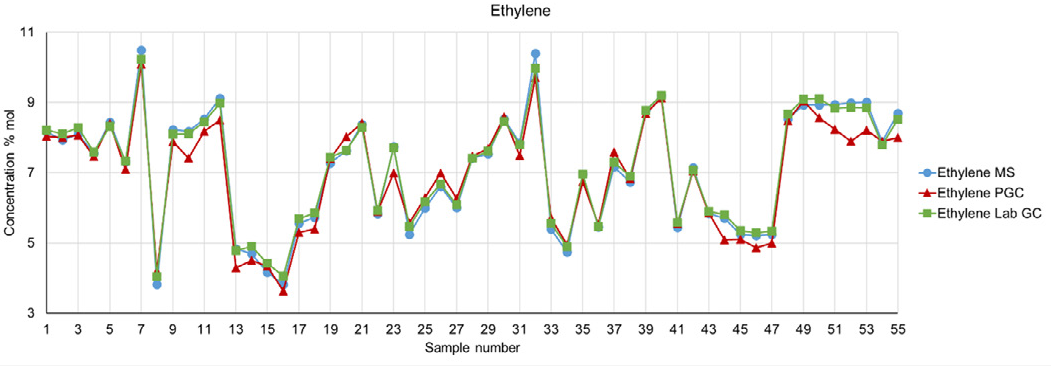
Figure 4. Ethylene data from MS, lab GC and process GC. Image Credit: Thermo Fisher Scientific – Environmental and Process Monitoring Instruments
Magnetic Sector MS Technology
The selection of MS technology is crucial because there are many different types of mass spectrometers, with each having its own distinct features. The data shown in this report demonstrates that they do not all share the same performance level. The scanning magnetic sector MS has been proven over the previous decades to have better analytical capabilities, as well as the ability to go longer periods between calibration and maintenance.
The scanning magnetic sector MS works by separating the positively charged ions that are generated from sample gas molecules in a variable magnetic field, before measuring the current generated by ions of each mass at a Faraday detector.
The spectral peaks produced in the magnetic field have a symmetrical shape with a flat top. The height of a peak directly corresponds to the component’s concentration, and its flat top ensures consistent height measurement while remaining tolerant of small variations in peak position. Figure 5 shows the design of a scanning magnetic sector MS.
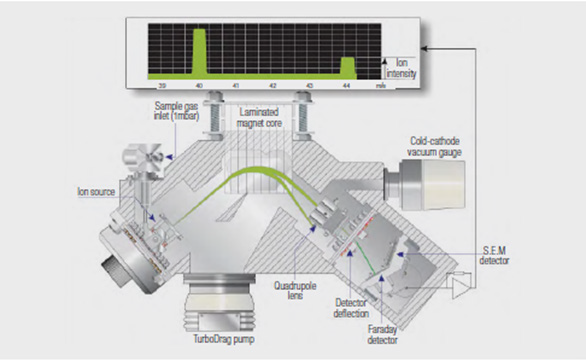
Figure 5. Scanning magnetic sector MS. Image Credit: Thermo Fisher Scientific – Environmental and Process Monitoring Instruments
Prima PRO Process Mass Spectrometer Performance Specifications
Thermo Scientific™’s latest model of process MS is the Prima PRO 710 Process Mass Spectrometer. In addition to being high-throughput, the magnetic sector MS is also highly accurate and capable of demonstrating precision over a wide range of concentrations. Table 1 shows the performance specifications for the Prima PRO710 MS when measuring a complex mixture of gases that are common to the polyethylene production process.
The cycle time for analysis is only 10 seconds: the addition of time for stream switching and purging makes the total cycle time for a multi-stream application approximately 20 seconds per point.
Table 1. Prima PRO 710 MS performance specification for polyethylene process gases. Source: Thermo Fisher Scientific – Environmental and Process Monitoring Instruments
|
Polyethylene process stream |
|
Concentration mol % |
Standard deviation mol % |
| Hydrogen |
1 |
≤ 0.01 |
| Ethylene |
7 |
≤ 0.05 |
| Ethane |
1 |
≤ 0.01 |
| Nitrogen |
3 |
≤ 0.05 |
| n-butane |
1 |
≤ 0.05 |
| 1-butene |
0.75 |
≤ 0.01 |
| Isobutane |
Balance |
≤ 0.05 |
| 1-hexane |
0.5 |
≤ 0.005 |
| n-hexane |
0.2 |
≤ 0.005 |
Summary
The Prima series process mass spectrometer is a well-suited system for monitoring process gases in the development of catalysts for the production of polyolefins. This technique is just as effective when scaled up to full-scale production. The data generated by the Prima series MS is ready in only a few seconds per sample stream and is highly accurate. By including a rapid multi-stream sampler (RMS), a single analyzer can be applied to multiple reactors, meaning this is a cost-effective system, with low operating and maintenance expenses.
The latest version of the Prima PRO 710 mass spectrometer, as well as its predecessors, have been used for many years in polyolefin development and manufacturing. Users who had previously used GC for these measurements report the following benefits of MS analysis:
- Narrower and more symmetrical distribution of polymer molecular weights
- More consistency in properties due to easier control of the comonomer
- Excellent reproducibility of tests
- Lower prevalence of unreliable trials
- Quicker output of experimental data
- Easier analytical operation with lower maintenance
Reference
- Towards Chem and Materials (2025) Polyolefin Market Volume to Reach 371.54 Million Tons by 2034. [online] Available at: https://www.towardschemandmaterials.com/insights/polyolefin-market.

This information has been sourced, reviewed, and adapted from materials provided by Thermo Fisher Scientific – Environmental and Process Monitoring Instruments.
For more information on this source, please visit Thermo Fisher Scientific – Environmental and Process Monitoring Instruments.