The hydrogen content of aviation fuel is an important parameter as it determines the combustion properties of the fuel. Traditional methods such as smoke point, smoke volatility index and luminometer number are tedious, time-consuming and usually require skilled analysts. Nuclear Magnetic Resonance (NMR) offers the opportunity to monitor the hydrogen content of fuels rapidly, non-destructively and with minimal sample preparation.
Advantages of Using NMR to Determine Hydrogen Content in Fuels
Advantages of Benchtop NMR include:
- NMR is a very stable technique over the long-term and therefore requires little re-calibration
- Minimal sample preparation is required
- The NMR technique is non-destructive, so repeatability measurements can be made conveniently
- Sample measurement time is relatively short
Determining Hydrogen Content in Fuels using NMR
For 20 years, Oxford Instruments Magnetic Resonance led the way with the Oxford Instruments Magnetic Resonance 4000 Continuous Wave (CW) NMR Analyser, an American Society for Testing and Materials (ASTM) compliant instrument, for rapid and efficient measurement of hydrogen content in fuels.
Since CW instruments are no longer commercially available the previous ASTM standard method has been updated for the use of Pulsed NMR.
In this method, the fuel samples are carefully transferred into glass tubes using a pipette, weighed and conditioned at 35°C or 40°C for 30 minutes prior to NMR analysis.
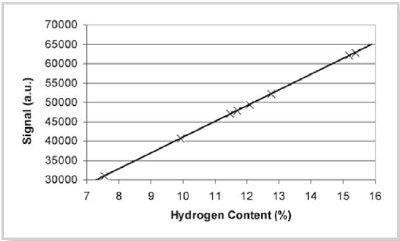
Figure 1: Hydrogen content calibration using hydrocarbons at 40°C
Although this method was designed for aviation fuel, it can be adapted to suit distillates covered by other methods (e.g. D3701-01 and D4808-01) as well as those which are more volatile or have a high wax content.
Calibration and Results
The instrument can be calibrated using real samples of known hydrogen content which span the range of interest; a list of chemicals are recommended in the standard method. In this example, the calibration was produced by using known masses of dodecane, diethyl malonate, cyclohexyl acetate, ethyl heptanoate, octyl acetate, ethyl caprate, 2-nonanone and pentadecane giving a correlation coefficient of 1.00 and standard deviation of 0.03. The predicted NMR results from this calibration are compared against the reference values in Table 1.
Table 1. Accuracy for the hydrogen in fuel method which is primarily dependent on sample preparation
| Sample |
Ref. %wt H
|
NMR %wt H
|
Difference
|
| Dodecane |
7.552
|
7.553
|
-0.001
|
| Diethyl malonate |
9.924
|
9.946
|
-0.022
|
| Cyclohexyl acetate |
11.466
|
11.510
|
-0.044
|
| Ethyl heptanoate |
11.703
|
11.709
|
-0.006
|
| Octyl acetate |
12.077
|
12.103
|
-0.026
|
| Ethyl caprate |
12.756
|
12.749
|
+0.007
|
| 2-nonanone |
15.185
|
15.227
|
-0.042
|
| Pentadecane |
15.386
|
15.385
|
+0.001
|
The precision of the experiment was checked by measuring a sample of 2- nonanone (12.756 %wt H) against this calibration curve, the results of which are shown in Table 2.
Table 2. Precision of the hydrogen in fuel method
|
Repeat
|
H Content (%)
|
|
1
|
12.757
|
|
2
|
12.757
|
|
3
|
12.752
|
|
4
|
12.739
|
|
5
|
12.730
|
|
6
|
12.737
|
|
7
|
12.745
|
|
Average
|
12.745 ± 0.010
|
The results show that the method gives accurate and reproducible measurement of hydrogen content in fuels.
Advantages of the Oxford Instruments Magnetic Resonance MQC-23 over Other Instruments
The MQC-23 with 0.55 Tesla magnet, fitted with an 18mm diameter (8 ml) sample probe is ideal for this application. The Hydrogen in Fuel package comprises:
- The MQC-23 which can be controlled using its own built-in computer using Microsoft® Windows® or via a stand alone PC
- MultiQuant software including RI Calibration, RI Analysis, and the EasyCal 'Hydrogen in Fuel' application
- 18 mm glass tubes
- PTFE stoppers (to seal the test cells)
- Stopper insertion/removal rod
- Installation manual
- Method sheet
In addition you may also wish to purchase:
- A dry heater and aluminium block with holes for conditioning the sample at 35°C or 40°C
- A precision balance
MQC-23 offers multiple advantages over other instruments on the market:
- High signal sensitivity
- Small benchtop footprint
- Low maintenance
- Minimal sample preparation

This information has been sourced, reviewed and adapted from materials provided by Oxford Instruments Magnetic Resonance.
For more information on this source, please visit Oxford Instruments Magnetic Resonance.