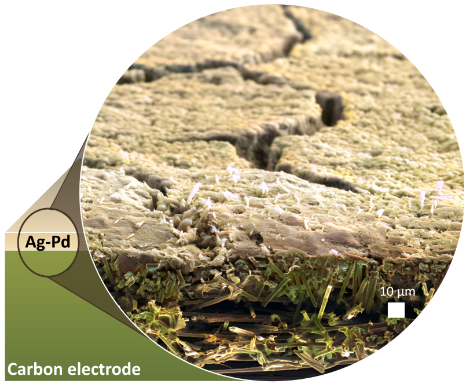
An illustration of a silver-palladium thin film deposited on a porous carbon electrode, which researchers believe could make hydrogen fuel cells easier and less expensive to manufacture. Image Credit: José Zamora Zeledόn, John Douglin, and Michaela Burke Stevens
This is particularly crucial to address the intermittent nature of renewable sources, ensuring a consistent power supply even during periods when sunlight and wind are not readily available.
The hydrogen fuel cell, a prominent candidate for energy storage, has received significant advancement through foundational research conducted at the Department of Energy's SLAC National Accelerator Laboratory and Stanford University.
This research, in collaboration with the Toyota Research Institute (TRI), has now been implemented in a practical fuel cell device through a partnership between Stanford and the Technion Israel Institute of Technology.
Hydrogen fuel cells have really great potential for energy storage and conversion, using hydrogen as an alternative fuel to, say, gasoline. But it's still fairly expensive to run a fuel cell.
Michaela Burke Stevens, Senior Author and Associate Scientist, SLAC
Michaela Burke Stevens is also associated with Stanford University's joint SUNCAT Center for Interface Science and Catalysis.
The challenge, as identified by Burke Stevens, lies in the fact that fuel cells traditionally depend on a catalyst containing costly platinum group metals (PGM) to enhance the chemical reaction essential for the system's functioning. This prompted Stevens and her colleagues to explore methods of creating a more cost-effective catalyst.
However, altering the fundamental chemistry of a fuel cell poses a significant challenge, as catalysts proven effective in laboratory settings may not necessarily perform well when implemented in real-world fuel cells by companies.
In their latest efforts, the researchers struck a balance in costs by substituting a portion of the expensive platinum group metals (PGMs) with a more cost-effective alternative, silver. However, the crucial aspect was simplifying the chemical formulation used to apply the catalyst to the electrodes of the fuel cell. Typically, scientists mix the catalyst into a liquid and then apply it to the mesh electrode.
The challenge lies in the variability of how these catalyst recipes perform in different laboratory environments with distinct tools, making it challenging to seamlessly transition the work into real-world applications.
Wet chemical processes are not particularly resilient for laboratory conditions.
Thomas F. Jaramillo, Department of Chemical Engineering, Stanford University
Thomas F. Jaramillo is also the Director at SUNCAT Center for Interface Science and Catalysis.
To circumvent this issue, the SLAC team opted for a different approach by utilizing a vacuum chamber for more precise depositions of their novel catalyst onto the electrodes.
This high-vacuum tool is a very ‘what you see is what you get’ type of method. As long as your system is calibrated well, in principle, people can reproduce it readily.
Thomas F. Jaramillo, Department of Chemical Engineering, Stanford University
To guarantee the replicability of their approach and its direct applicability to full-scale fuel cells, the team collaborated with experts at Technion. The Technion team demonstrated that the method is effective in a practical fuel cell setting.
“This project was not set up to do the fuel cell testing here, so we were really fortunate that the lead Stanford graduate student on the project, José Zamora Zeledόn, formed a connection with Dario Dekel and his PhD student John Douglin at Technion. They were set up to test the actual fuel cells, so it was a really nice combination of resources to put together,” adds Michaela Burke Stevens.
Collaboratively, the two teams discovered that by replacing some of the costly PGMs with more affordable silver in previous catalyst formulations, they produce an equally efficient fuel cell at a significantly reduced cost. With a validated method for catalyst development in place, they can now explore more ambitious ideas and innovations. “We could try going entirely PGM-free,” said Jaramillo.
“This has great benefits for the research of fuel cells in the academy as well as for practical catalyst development in the fuel cell industry,” says Jaramillo. Dekel, a chemical engineering Professor and Director of the Grand Technion Energy Program at Technion.
Moving ahead, Jaramillo emphasized that research endeavors similar to this one will play a crucial role in determining whether fuel cells can fulfill their potential. Jaramillo adds, “Fuel cells are really looking exciting and interesting for heavy-duty transportation and clean energy storage. But it’s ultimately going to come down to lowering cost, which is what this collaborative work is all about.”
The study was funded in part by the DOE's Office of Science through the SUNCAT Center for Interface Science and Catalysis, a SLAC-Stanford joint institute, and the Toyota Research Institute.
Journal Reference
Douglin, C, J., et al. (2023). High-performance ionomerless cathode anion-exchange membrane fuel cells with ultra-low-loading Ag–Pd alloy electrocatalysts. Nature Energy. doi/s41560-023-01385-7