Magritech’s Spinsolve NMR Benchtop is the ideal teaching tool of NMR for undergraduate education. The device has sophisticated features and excellent sensitivity implying it is quick and informative and crucial for a busy class. Spinsolve offers high convenience, excellent performance NMR as a much lower cost when compared to traditional NMR.
Spinsolve Features High Speed
ñ-nitroaniline is synthesized by hydrolyzing ñ-nitroacetanilide in one second year experiment by students. Within just a few minutes of sample preparation, Spinsolve NMR spectra were obtained. On a standard 300Mz instrument, comparison measurements were also made.
Figure 1 shows the 1H NMR spectra of 200mM solutions of starting material indicated in red and the final product in blue in DMSO-d6. The reaction is successful when the methyl peak CH3 disappears and the NH peak converts from 10.5ppm to NH2 at 6.6ppm. It can be seen from the results that by using a lower NMR frequency, there is no loss of chemical information.
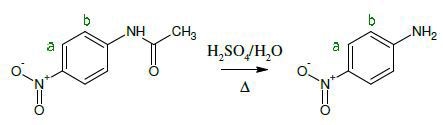
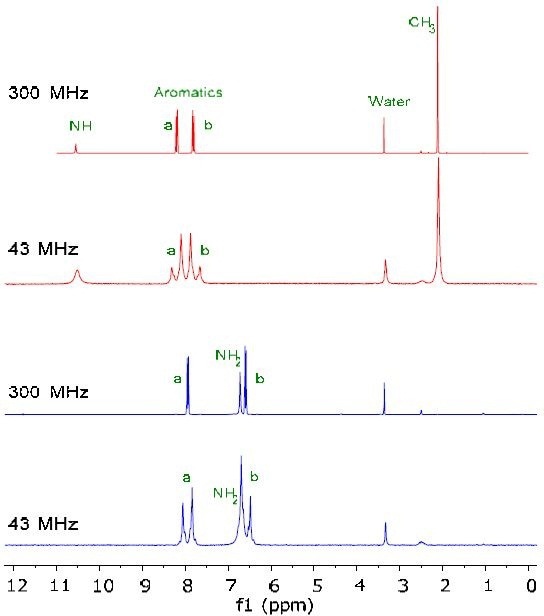
Figure 1
The high-speed benefits of Spinsolve are:
- Spinsolve is rapid i.e students obtain informative spectra within a minute of sample preparation
- It is convenient as it is in the lab, on the bench, next to the students
- Due to excellent sensitivity, students can work with concentrations of tens to hundreds of mmol
- Spinsolve 43 MHz spectra offer similar chemical information as high field.
Spinsolve Features Low Cost
As costly deuterated solvents are not needed, Spinsolve reduces costs and is less expensive than high-field NMR. In this second year chemistry experiment, ñ-nitroacetanilide is synthesized by students by nitrating acetanilide.
The NMR spectra of starting material (red) and final (blue) product is shown in Figure 2 in deuterated and non-deuterated DMSO. It is possible to still make all peak assignments for the molecule even though there is a large peak for the non-deuterated solvent.
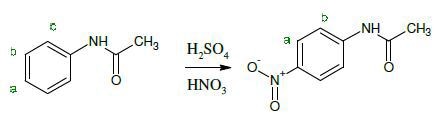
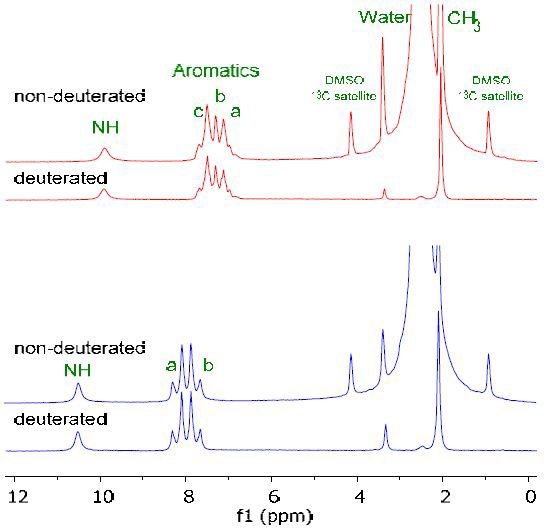
Figure 2
The cost benefits of Spinsolve are:
- Deuterated solvents are not necessary
- Low cost budget NMR tubes can be used
- Samples can be analyzed in native solutions
- Solubility issues are resolved - any solvent can be used
- Enables in-line monitoring of reactions.
Spinsolve Features Easy Operation
The Spinsolve features a convenient user interface that enables even a novice to easily use the system. For instance, a number of inorganic complexes are paramagnetic ions. The chemical shift range of proton spectra is extended to hundreds of ppm by these ions. The Spinsolve software is especially tailored for scanning such samples and can be operated easily. A third year experiment shows coordinated ligand reaction using conversion of paramagnetic [Co(phen) 3]2+ to diamagnetic [Co(phen3)]3+ complexes.
The drastic difference of the chemical shift range between the diamagnetic and paramagnetic complexes is shown in Figure 3.
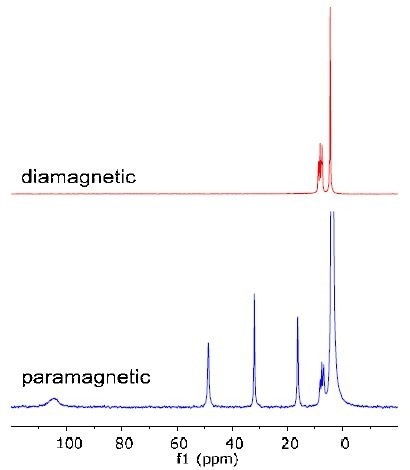
Figure 3
The user interface is highly convenient and offers the following benefits:
- Students can perform different experiments using the simple to use interface
- Students can also measure 19F spectra with the click of a button
- Paramagnetic samples can be analyzed easily
- Advanced 2D NMR experiments are simple to run
Advanced NMR-COSY Experiment
It is difficult for students to understand and differentiate between chemical shift and j-coupling when they are first exposed to NMR. 2-D NMR experiments project added information to a second dimension that enables the interpretation of NMR spectra.
As an example, the 1H spectrum of ibuprofen dissolved in CDCl3 is considered. In order to assign which proton resonances are coupled to each other, the COSY experiment is used. Through-bond coupling is indicated in the cross peaks in the 2D spectrum. Figure 4 shows the COSY spectrum for ibuprofen. Two spin systems are observed from the cross peaks - CH-2/CH3-10 and CH2-7/CH3-9.
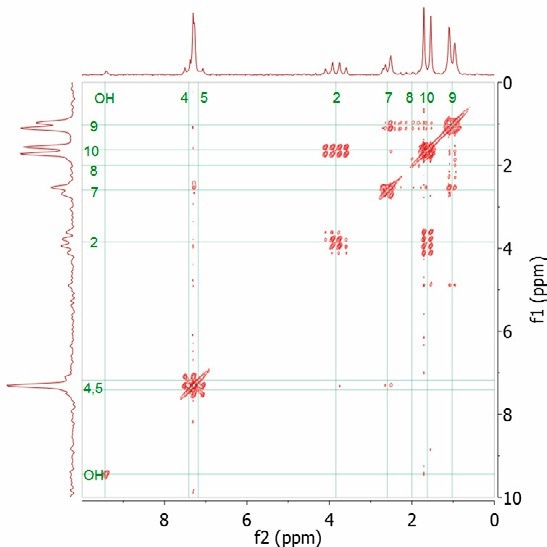
Figure 4
The key features of the COSY experiment are:
- The COSY experiment identifies the proton signals from magnetically coupled chemical groups
- 2D COSY is one of the most commonly used 2D NMR experiments
- Takes 10 minutes to run, ideal for a teaching laboratory
Intuitive Software
Spinsolve software is highly intuitive and cab ne operated by anyone in the lab with little or no training. With just one click of a button, most experiments can be run. Also switching between experiments can be done at the click of a button.
Figure 5 shows a screenshot of the new 19F capability built into the latest version of the Spinsolve software.
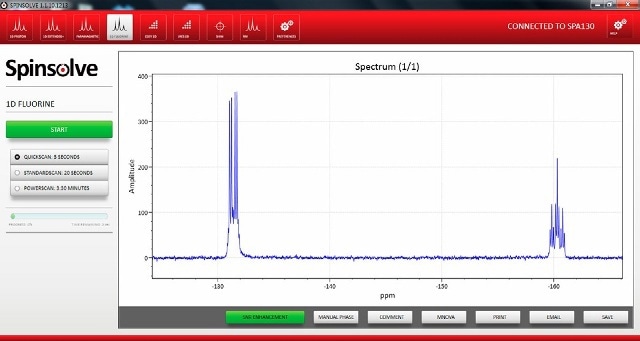
Figure 5
Benefits of Spinsolve in Education
The Spinsolve is less expensive and it is possible to use non-deuterated solvents. Budget NMR tubes can be used and features a low power consumption. Budget NMR tubes can be used. Spinsolve fits easily on a laboratory bench and features simple and intuitive software. It is safe as there is no stray magnetic field. It helps obtain high-resolution NMR data in below 10s. It is offered with 19F fluorine, 2D and multi-pulse experiments. Students and teaching staff gain hands-on experience with NMR.
This information has been sourced, reviewed and adapted from materials provided by Magritek.
For more information on this source, please visit Magritek.