It is very important to separate and purify products from synthetic reaction mixtures. Extraction methods are used for separating the desired product from un-reacted starting materials or from undesired side products in the reaction mixture.
The article covers an experiment wherein a mixture containing an acid, base and a neutral compound need to be separated using acid or base extraction. The three compounds that need to be separated are:
Dichloromethane is used as the extraction solvent as shown in Figure 1. The compound will also be purified and the percent recovery of each compound from the mixture will be determined.
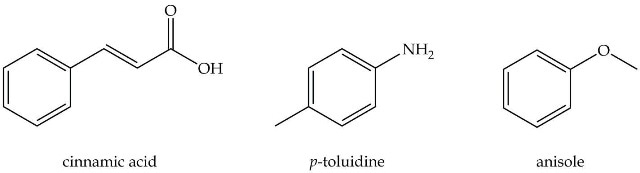
Figure 1. Organic compounds to be separated.
Experimental Section
Safety
These precautions need to be followed:
- Sodium hydroxide and hydrochloric acid feature high corrosion, need to be used carefully and the experiment must be done in a fume hood with the protective glass door pulled down.
- Dichloromethane and p-toluidine are toxic and it is important to avoid contact with the skin, eyes and clothes.
- Cinnamic acid is an irritant.
- Anisole is flammable and may cause eye and skin irritation.
Separation Procedure
The separation procedure is as follows:
- Obtain and weigh a sample containing 1g cinnamic acid, 1g p-toluidine and 6mL anisole.
- The sample is dissolved in 35mL dichloromethane (35mL) and the mixture is transferred to a separatory funnel.
- Take a 1ml aliquot for 1H NMR. The mixture is extracted with hydrochloric acid (2x15mL, 2M). The basic compound p-toluidine alone will react to form the ionic compound, p-toluidinium chloride, which will dissolve in the aqueous layer and is removed.
- Next take an aliquot (1mL) of the aqueous layer for 1H NMR. The rest of the dichloromethane layer contains cinnamic acid and anisole.
- For 1H NMR, take 1mL aliquot of this layer. The dichloromethane layer is basified with sodium hydroxide solution (2x15mL, 2M). Sodium cinnamate is then formed that will dissolve in the aqueous layer and is removed.
- 1mL aliquot of the aqueous layer is taken for 1H NMR. The remaining dichloromethane layer now only contains the neutral compound anisole.
- Take an aliquot (1mL) of this layer for 1H NMR. These operations are conveniently represented in a flow diagram as shown in Figure 2.
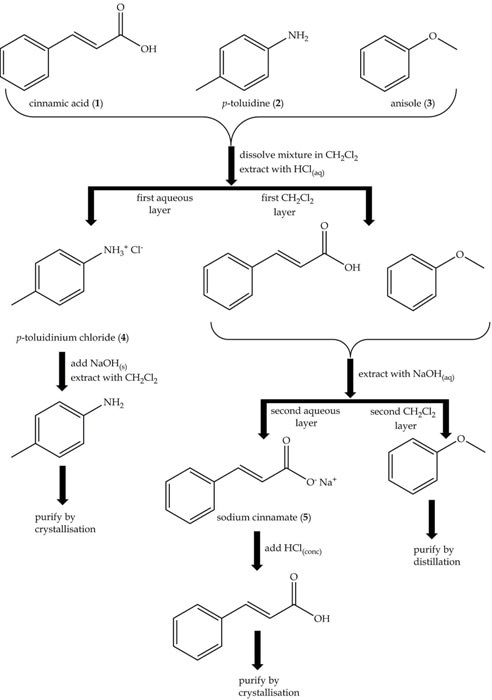
Figure 2. Flow diagram for the separation of an acid, a base and a neutral compound: cinammic acid (1), p-toluidine (2) and anisole (3).
Purification Procedures
Isolation and Purification of Cinnamic Acid
The aqueous layer containing the ionic compound sodium cinnamate is acidified with concentrated HCl. The solution is tested with litmus paper to confirm its acidity. The precipitate is collected by filtration then recrystallized freom hot water. The pure cinnamic acid is filtered, dried in the air and yield is recorded.
Isolation and Purification of p-toluidine
For regeneration of p-toluidine, the aqueous layer containing the ionic compound p-toluidinium chloride is basified with sodium hydroxide pellets. The solution is tested with litmus paper to confirm its basicity. The basic solution is transferred to a separatory funnel and extracted with dichloromethane (2x20mL). The top aqueous layer is discarded. The dichloromethane layer is dried with anhydrous sodium sulfate (Na2SO4), and then an aliquot (1mL) is taken for 1H NMR.
Isolation and Purification of Anisole
In order to purify crude anisole, the dichloromethane is removed on a rotary evaporator and the 1H NMR spectrum of crude anisole is recorded as a neat liquid. The 1H NMR spectrum (Figure 3) of the initial separation mixture in dichloromethane shows the presence of all the three compounds to be separated.
At 2.36ppm, a singlet is observed based on the methyl group of p-toluidine. Also, downfield (higher ppm), another singlet is observed at 3.87ppm with regards to the OCH3 group of anisole. The peaks at 8.13 and 7.75 ppm make a doublet related to one of the alkene protons of cinnamic acid. A complex multiplet is observed between 6.5-7.6 ppm for the aromatic protons of all three compounds. There are 13C satellites from the methylene chloride solvent.
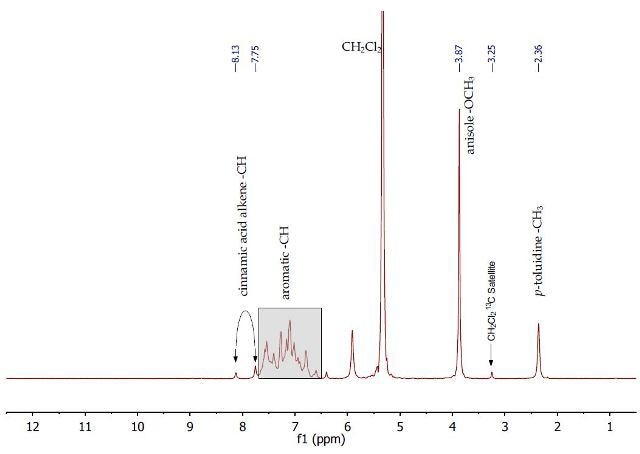
Figure 3. 1H NMR spectrum of initial separation mixture containing cinnamic acid, p-toluidine and anisole in dichloromethane
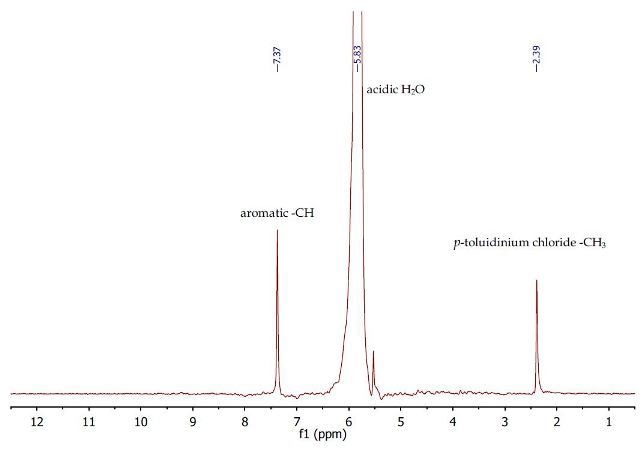
Figure 4. 1H NMR spectrum of the first aqueous layer containing p- toluidinium chloride
The 1H NMR spectrum (Figure 4) of the first observed at 2.39 and 7.37ppm, with regards to the aqueous layer shows the presence of only the p-toluidinium chloride methyl group and the p- toluidinium chloride. Two singlets are aromatic protons, respectively.
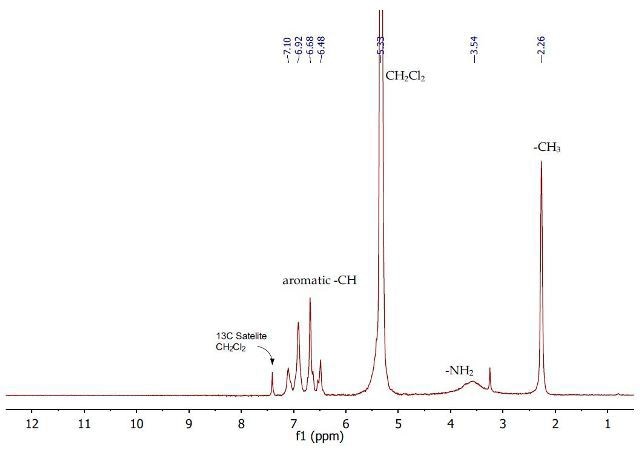
Figure 5. 1H NMR spectrum of regenerated p-toluidine (2) in dichloromethane.
Figure 5 shows the 1H NMR spectrum of the dichloromethane layer obtained from the addition of sodium hydroxide to the first aqueous layer containing p-toluidinium chloride. The 1H NMR spectrum shows that p-toluidine has been regenerated.
At 2.26ppm, a singlet is observed with regards to the p-toluidine methyl group, a very broad singlet is observed for the NH2 protons at 3.54ppm and a multiplet is observed between 6.48-7.10ppm for the aromatic protons. This p-toluidine sample has a high level of purity as this spectrum is the same as the spectrum is identical to the spectrum of pure p-toluidine after crystallisation.
It can be seen from the 1H NMR spectrum in Figure 6 of the first dichloromethane layer, that only cinnamic acid and anisole is present as p-toluidine has been removed after extraction with aqueous HCl. Only one singlet is observed at 3.83ppm corresponding to the OCH3 group of anisole.
A complex multiplet is observed between 7.8-7.6 ppm for the aromatic protons of both compounds. Both alkene CH proton doublets of cinnamic acid are now resolved at 7.7/8.1 and 6.3/6.7ppm. These two protons are coupled with a J-coupling constant of about 16Hz.
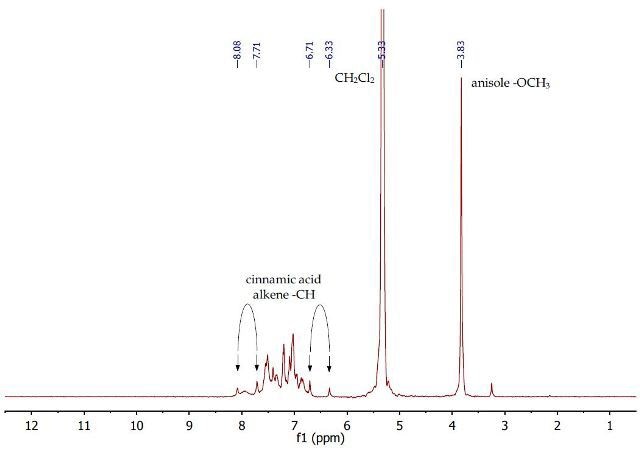
Figure 6. 1H NMR spectrum of second dichloromethane layer containing anisole and cinnamic acid in dichloromethane.
The 1H NMR spectrum (Figure 7) of the second dichloromethane layer shows the presence of only anisole, as cinnamic acid (1) was removed after extraction with aqueous NaOH. A singlet is observed at 3.81ppm with regards to the OCH3 group of anisole, and a complex multiplet is observed between 6.81-7.51ppm for the aromatic protons.
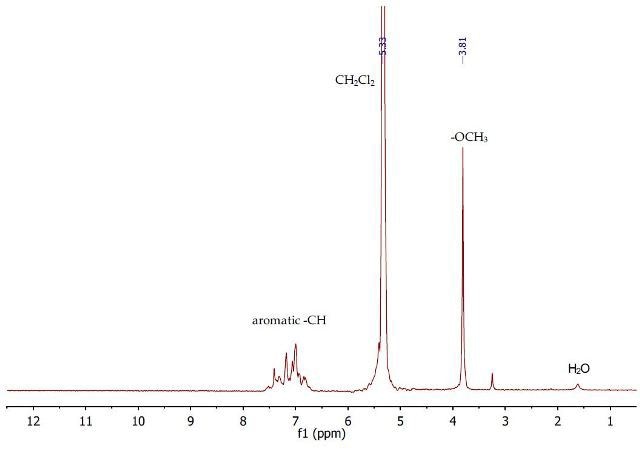
Figure 7. 1H NMR spectrum of the second dichloromethane layer containing anisole
The 1H NMR spectrum (Figure 8) of crude anisole after the removal of dichloromethane clearly shows that the separation procedure was very effective at separating the neutral compound from the mixture. The spectrum shows a singlet at 2.89ppm corresponding to the OCH3 group of anisole, and a complex multiplet between 6.12-6.69ppm for the aromatic protons. This anisole sample has a high degree of purity as this spectrum is identical to the spectrum of pure anisole after distillation.
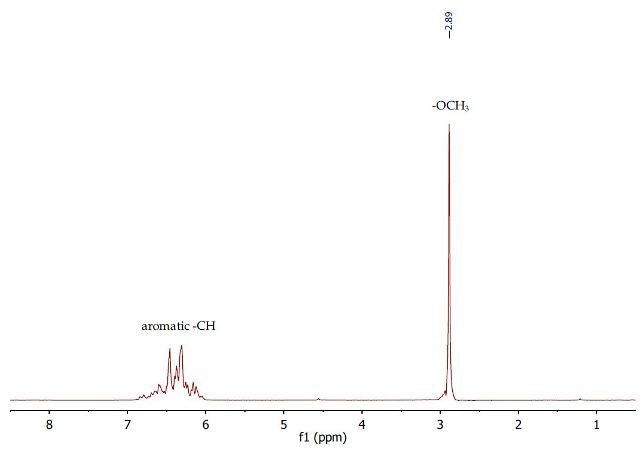
Figure 8. 1H NMR spectrum of crude anisole
The 1H NMR spectrum (Figure 9) of the second aqueous layer shows the presence of the ionic compound sodium cinnamate after extraction with aqueous NaOH. The aromatic protons and the alkene CH protons of sodium cinnamate are observed between 6.2 and 7.5ppm. The down field CH proton peak, corresponding to the protons closer to the carboxylate, has now shifted into the aromatic multiplet.
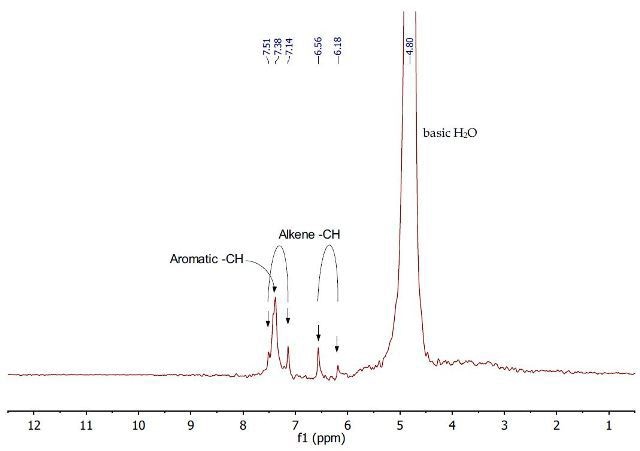
Figure 9. 1H NMR spectrum of the second aqueous layer containing sodium cinnamate
The 1H NMR spectrum (Figure 10) of purified cinnamic acid in dichloromethane shows a multiplet between 7.41-7.52ppm for the aromatic protons. The alkene CH protons are observed as doublets (3Jhh = 16.1 Hz) at 6.47 and 7.85 ppm. The OH proton is also observed at 12.02 ppm as a broad singlet.
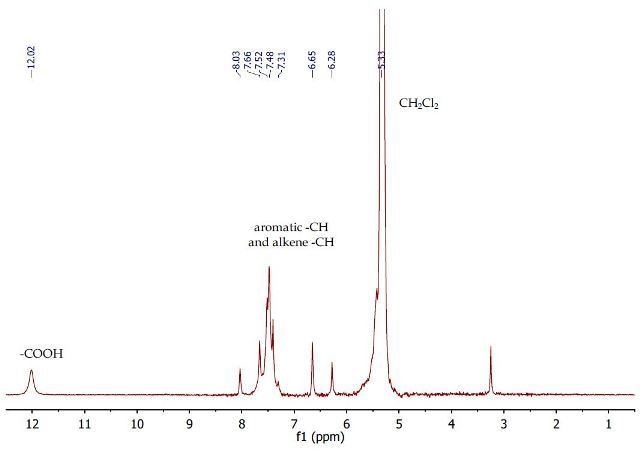
Figure 10. 1H NMR spectrum of cinnamic acid in dichloromethane
About Magritek
Founded in 2004, Magritek is an advanced technology company exporting from Germany and New Zealand to customers all over the world. The initial technology and IP used in Magritek products was developed by research teams at RWTH Unviersity, Germany, and Massey University and Victoria University of Wellington in New Zealand.
Today, Magritek provides complete NMR and MRI system solutions for the oil and gas industry, as well as components and subsystems suitable for research laboratories and education.
This information has been sourced, reviewed and adapted from materials provided by Magritek.
For more information on this source, please visit Magritek.