
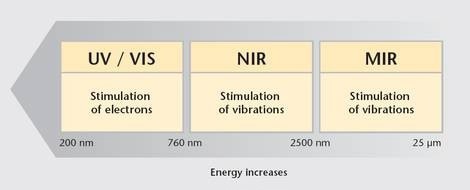
Optical spectroscopy covers a wavelength range of 200 nm to 25 µm (Figure 1), and is divided into three important spectral ranges:
- Ultraviolet/visible: 200 nm – 760 nm
- Near-Infrared (NIR): 760 nm – 2500 nm
- Mid-Infrared: 2500 nm – 25 µm
Photons in the ultraviolet/visible spectral range have sufficient energy to ionize or excite materials by increasing the energy level of bound electrons. NIR radiation has less energy per photon but does excite molecular vibrations.
Theory of NIR Spectroscopy
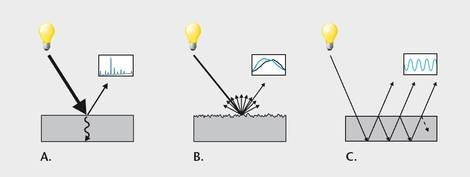
The interaction between the sample and the electromagnetic radiation can be explained by the following effects:
- Absorbance/Reflection (Figure 2a)
The absorbance spectra are employed for the quantitative and qualitative determination of composition.
- Surface effects (Figure 2b)
The evaluation of surface effects determines the physical characteristics such as grain size and roughness.
- Interface effects (Figure 2c)
Interference patterns in returned spectra can be employed to estimate the thickness of thin layers.
Spectra Evaluation Methods
Chemometrics involves the application of statistical techniques (e.g., partial least squares or principal components analysis) to extract information from spectroscopic or chemical data. There are quantitative and qualitative methods for performing multi-component analysis. Quantification, such as linking sensor signal with substance concentration, can be done even with no specific interactions.
Fields of Application
The wavelength of light used in NIR spectroscopy excites vibrations of covalent molecular bonds. Hence, it can be used to determine water content in agrochemical and food products (feedstuff, renewable raw materials, milk, and corn). Pharmaceutical and organic products can be examined with respect to their fat content (C-H bonds) or protein (N-H bonds). Additionally, various molecular structures and groups can be identified in polymers. A perfect example is given by the carboxylic groups (COOH). For these reasons, the method is usually employed in the pharmaceutical, chemical, and food industries for process control and quality assurance.

This information has been sourced, reviewed and adapted from materials provided by Polytec.
For more information on this source, please visit Polytec.