Table of Contents
Introduction
Sample
Sample Preparation
Columns
Solutions
Parameters
Combustion Parameters
Analysis
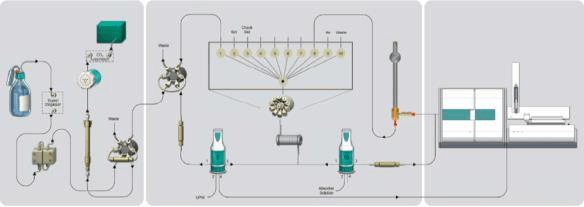
Instrumentation
Results
Introduction
Bromination of polystyrol is carried out to increase flame retardation. The resulting brominated polystyrene is composed of 25% to 35% of bromine.
For bromine determination using combustion ion chromatography (CIC), a specially optimized absorption solution is required to trap all bromide. This article covers a work showing the optimization of the absorption solution for high-bromine samples.

 Download the Article
Download the Article
Sample
Brominated polystyrene
Sample preparation
The sample analysis is carried out using Combustion IC with flame sensor technology and intelligent partial loop injection technique with inline matrix elimination.
Columns
|
Metrosep A Supp 16 - 100/4.0
|
6.1031.410
|
|
Metrosep A Supp 4/5 Guard/4.0
|
6.1031.500
|
|
Metrosep A PCC 1 HC/4.0
|
6.1006.310
|
|
Metrosep A Trap 1 - 100/4.0
|
6.1014.000
|
|
Metrosep I Trap 1 - 100/4.0
|
6.1014.200
|
Solutions
|
Eluent
|
7.5 mmol/L sodium carbonate
0.75 mmol/L sodium hydroxide
|
|
Suppressor regenerant
|
250 mmol/L sulfuric acid
|
|
Rinsing solution
|
STREAM
|
|
Absorber solution
|
100 - 800 mg/L hydrogen peroxide
0 - 30 mmol/L sodium hydroxide
|
Parameters
|
Flow rate
|
0.7 mL/minutes
|
|
Injection volume (IC)
|
10 μL (MiPT)
|
|
Pmax
|
15 MPa
|
|
Recording time
|
14 minutes
|
|
Column temperature
|
45 °C
|
Combustion parameters
|
Argon
|
100 mL/minutes
|
|
Oxygen
|
300 mL/minutes
|
|
Oven temperature
|
1050 °C
|
|
Post-combustion time
|
600 s
|
|
Initial volume of absorption solution
|
4.0 mL
|
|
Water inlet
|
0.2 mL/minutes
|
Analysis
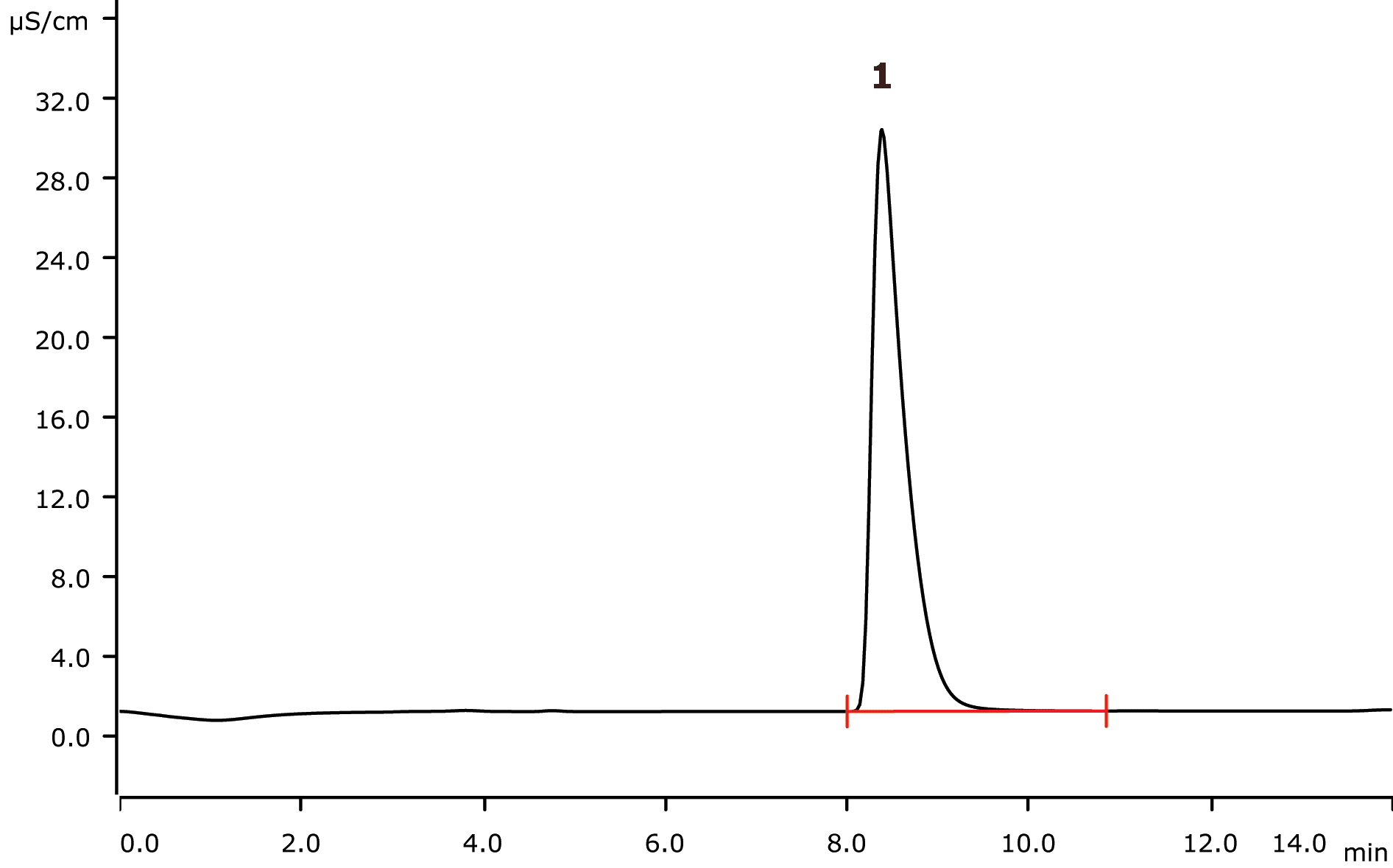
Conductivity after the sequential suppression
Instrumentation
|
930 Compact IC Flex Oven/SeS/PP/Deg
|
2.930.2560*
|
|
IC Conductivity Detector
|
2.850.9010*
|
|
MSM Rotor A
|
6.2832.000*
|
|
Adapter sleeve for Suppressor Vario
|
6.2842.020*
|
|
920 Absorber Module
|
2.920.0010*
|
|
Combustion Module (oven and ABD)
|
2.136.0700*
|
|
Autosampler MMS 5000
|
2.136.0800
|
|
Kit for solid sampling
|
6.7302.000
|
* Available as 930 Metrohm Combustion IC (2.930.9010)
Results
|
1 Bromine
|
NaOH [0 mol/L]
|
NaOH [10 mol/L]
|
NaOH [20 mol/L]
|
NaOH [30 mol/L]
|
|
Change in NaOH
(400 mg/L H2O2)
|
241.0 g/kg
RSD = 4.9%
|
287.6 g/kg
RSD = 3.6%
|
287.6 g/kg
RSD = 1.5%
|
283.8 g/kg
RSD = 1.8%
|
|
|
H2O2 [100 mol/L]
|
H2O2 [200 mol/L]
|
H2O2 [400 mol/L]
|
H2O2 [800 mol/L]
|
|
Change in H2O2
(20 mmol/L NaOH)
|
288.2 g/kg
RSD = 2.8%
|
270.6 g/kg
RSD = 2.0%
|
281.5 g/kg
RSD = 1.8%
|
284.8 g/kg
RSD = 2.9%
|

This information has been sourced, reviewed and adapted from materials provided by Metrohm AG.
For more information on this source, please visit Metrohm AG.