Electron ionization (EI) is a hard ionization method that is typically used with gas chromatography-mass spectrometry (GC-MS). The mass spectral fragmentation patterns generated by EI are used for library database searches to determine compounds.
On the contrary, soft ionization methods such as field ionization (FI) typically produce clear molecular ions with the lowest fragmentation. When utilizing high-resolution MS in combination with these ionization methodologies, the accurate masses for the fragment ions generated by EI and the molecular ions produced by soft ionization providing a further dimension of information for the analytes. Compounding the precise mass information with the results of traditional library searches can improve the accuracy of identification in contrast to the use of library search alone.
This study presents the msFineAnalysis software and uses it to automatically merge data obtained by GC/EI and GC/soft ionization for the qualitative analysis of compounds produced by the pyrolysis of a vinyl acetate resin.
Experimental
Using a commercial vinyl acetate resin was used as a model polymer sample, the JEOL JMS-T200GC was fitted with a combination of EI/FI ion source for the analysis. A Frontier 3030D pyrolysis unit was set up on the GC inlet and utilized for pyrolyzing the sample. Table 1 illustrates the measuring conditions of the pyrolysis GC/TOFMS.
Table 1. Measuring conditions.
| . |
. |
| Pyrolysis Conditions |
Pyrolysis Temp.: 600 °C |
| GC Conditions |
Column: DB-5ms Ul, 15 m x 0.25 mm, 0.25 µm
Oven temp.: 50 °C (1 min)→30 °C /min→330 °C (1.7 min)
Injection mode: Split mode (100:1) |
| MS Conditions |
MS: JEOL JMS-T200GC
Ion source: El/FI combined ion source
Ionization: Eli+ (70 eV, 300 µA), Fl+ (-10 kV, 6 mA/10 msec (Carbotec)) |
The consequent EI and FI data were then analyzed automatically by using the new JEOL msFineAnalysis software. The subsequent report was then compared to traditional GC/EI qualitative analysis.
Qualitative Analysis Work Flow:
Figure 1 exhibits the operational flow chart for the structured analysis steps used for the JEOL msFineAnalysis software (chart on the right). Initially, the data was obtained by using EI and soft ionization (SI), and all peaks and connected mass spectra are determined in the chromatograms.
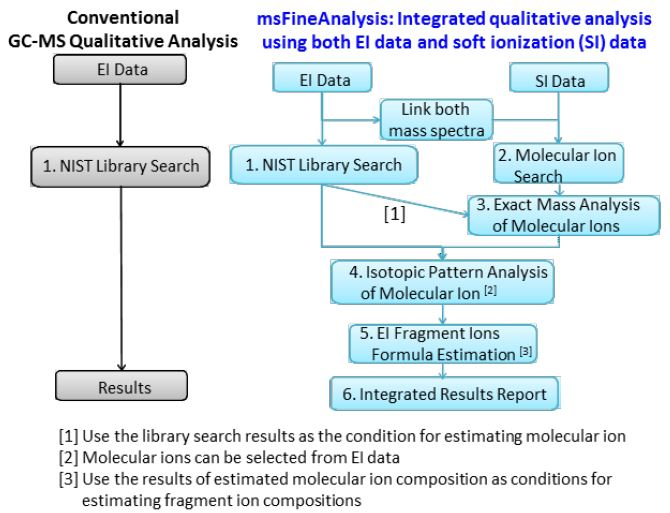
Figure 1. Comparison of conventional and msFineAnalysis qualitative analysis workflows
Thereafter, the mass spectra generated by these ionization methodologies are connected using their retention times, and these connected mass spectra are registered as single components. Following this, the EI mass spectrum is used for the library database search (1), and the SI mass spectrum is used to determine the analyte molecular ion (2).
Subsequently, for exact mass analysis, the molecular ion is used to approximate any possible elemental compositions, and these candidate formulas are then reduced by using the EI library search results (3). Next, the molecular ion is subject to isotopic pattern analysis to help further determine the candidate formulas (4).
Each candidate formula is then deployed as the search limits for the precise mass analysis of the EI fragment ions (5). If the molecular ion formula candidate is determined to be erroneous, the EI fragment ions will not produce many (if any) compositional formulas. Therefore, the indication is that the molecular ion formula is not an appropriate candidate for that specific analyte. These results are then exhibited as an integrated qualitative analysis report (6).
However, the traditional qualitative analysis workflow that is typically used for GC-MS is displayed on the left side of the chart. This process involves obtaining only the EI data and then conducting the necessary database searches for each analyte.
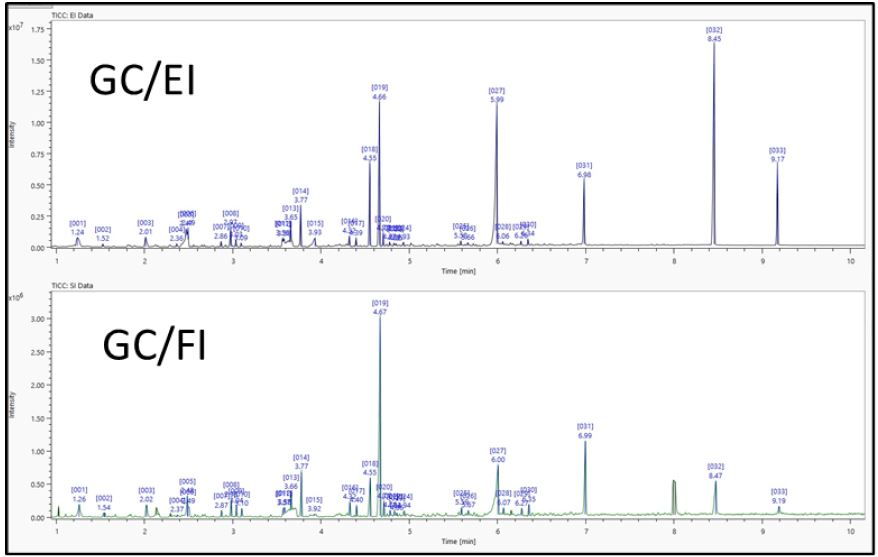
Figure 2. TIC chromatograms of vinyl acetate resin acquired by Py/GC/TOFMS.
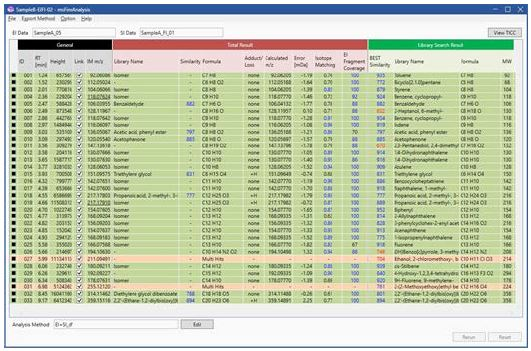
Figure 3. Integrated qualitative analysis report produced by msFineAnalysis.
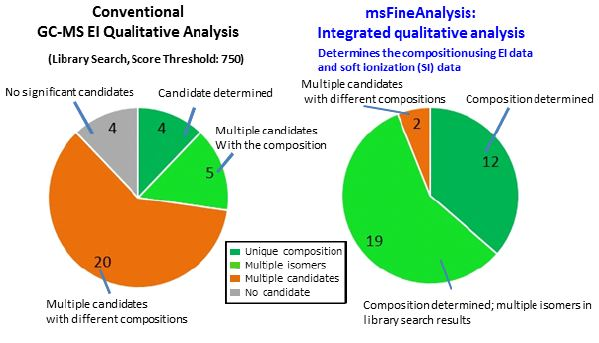
Figure 4. Comparison results. Left: Conventional GC-MS qualitative analysis. Right: An integrated qualitative analysis.
Results and Discussion
Operation of the msFineAnalysis Auto Analysis determined 33 components in the GC/EI and GC/FI measurements (Figure 2) that were systemically connected using their retention time. The Auto Analysis function then automatically performed the steps in Figure 1 to analyze the connected data, and the results were displayed as a color-coded table as illustrated in Figure 3. Each color signifies a level of conviction for the determination of the compound:
Green: A molecular formula candidate was uniquely determined.
Orange: Multiple molecular formula candidates were determined.
White: No significant molecular formula candidates were determined.
The components categorized as orange or white can be reviewed to a greater extent manually to determine a unique candidate formula. In this instance, the msFineAnalysis Auto Analysis function was able to unambiguously identify the molecular compositions for over 90% of the components, which is a considerable improvement over the traditional qualitative analysis method in which only ~1/4 of the observed components were determined with a unique molecular formula (Figure 4).
Furthermore, the EI fragment ion formulas, which were obtained synchronously, lead to additional structural information about the sample molecules that further endorsed the identified molecular composition.
Conclusions
The msFineAnalysis qualitative analysis method unites GC/EI and GC/FI precision data en masse to generate extremely accurate qualitative results. For the components recorded in library databases (match factor score: high), these results are cross-referenced with the precise mass molecular formula identification.
Furthermore, this software makes it feasible to approximate molecular formulas for unidentified components that are not recorded in the library database (match factor score: low). Typically, this process makes it difficult to determine when only the traditional analysis method is used (chart on the left of Figure 1).
Therefore, this integrated analysis method can determine molecular formulas and fragment ion formulas from exact masses, irrespective of the level of match factor score, in order to specify the formula candidates, consequently supplying an effective tool for GC/MS qualitative analysis.

This information has been sourced, reviewed and adapted from materials provided by JEOL USA, Inc.
For more information on this source, please visit JEOL USA, Inc.