Acid catalysts have long been a cornerstone of the chemical industry, ever since the initial development of plants designed to convert oil into valuable petrochemical products.
As the necessary shift towards sustainable resources accelerates, these older catalysts are no longer applicable.
This is because biomass-based compounds feature an array of different functional groups, each necessitating the use of a dedicated catalytic functionality to be effectively converted into their target products.
These demands for new functionalities mean that developing new catalytic materials is vital.
Recent studies have aimed to develop a series of zirconia catalysts modified with various silica loadings. These catalysts have demonstrated mixed acid-based properties, depending on the relative silica content.
An unexpected Brønsted type surge in acidity at a certain Zr/Si ratio was explicitly observed. This was mirrored by a shift in catalytic activity.
Using these acidic catalysts, it was deemed possible to convert biomass-derived molecules, such as HMF and furfural, into their corresponding ethers. These ethers are in high demand as biofuels.
As part of this process is catalyzed by the acidic functionalities of the catalysts, it was necessary to employ a probe reaction - the 2-propanol dehydration to propene - to assess these features.
Catalysts were impregnated with 2-propanol before raising their temperature under a continuous inert gas flow. A Hiden HPR-20 mass spectrometer enabled real-time analysis of the desorption of 2-propanol and the formation of propene, along with acetone as a possible side product.
These compounds’ main mass signals were recorded as a function of time before being correlated to temperature (Figure 1).
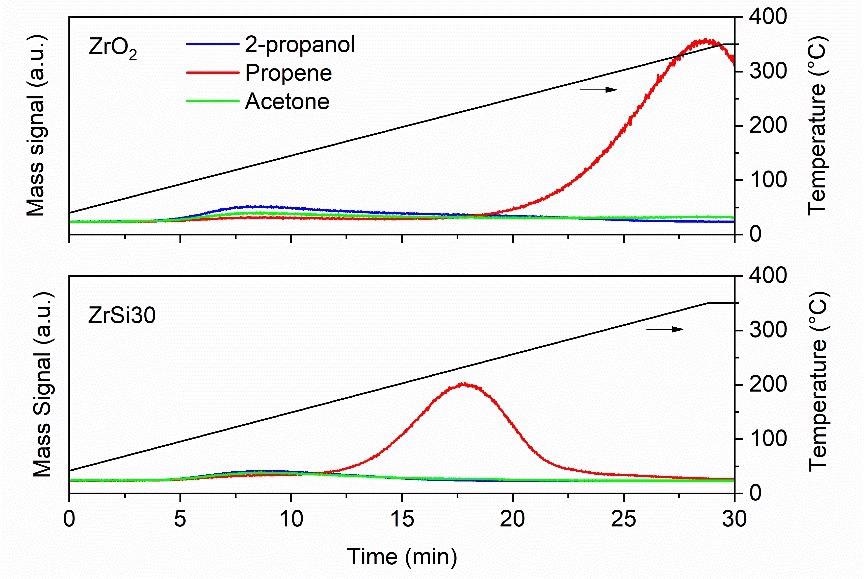
Figure 1. Comparison of the 2-propanol dehydration power of zirconia and zirconia-silica catalysts. Image Credit: Hiden Analytical
As anticipated, the catalyst containing a certain silica amount (ZrSi30) and possessing a more significant amount of Brønsted acid sites outperformed the bare zirconia by dehydrating the 2-propanol at a far lower temperature (220 °C versus 340 °C).
This is evident in the profile of the propene signal (Figure 1), whereby its maximum appears before that of the bare zirconia catalyst. This essentially means that the dehydration reaction was triggered at a lower temperature.
It was possible to rapidly assess the acid character of these and other materials thanks to a combination of the relative simplicity of this type of probe reaction and the Hiden HPR-20 mass spectrometer’s ease of use and versatility.
Reference
“On demand production of ethers or alcohols from furfural and HMF by selecting the composition of a Zr/Si catalyst” Catalysis Science & Technology, 2020 (10) 7502-7511 https://doi.org/10.1039/D0CY01427C
Acknowledgments
Produced from materials originally authored by Filippo Bossola from CNR, Istituto di Scienze e Tecnologie Chimiche “Giulio Natta” (SCITEC).

This information has been sourced, reviewed and adapted from materials provided by Hiden Analytical.
For more information on this source, please visit Hiden Analytical.