In this interview, Instron's Landon Goldfarb discusses the importance of mechanical testing for medical devices used in diabetes care and treatment.
How has the treatment of diabetes changed over the last 20 years?
The treatment of diabetes has mirrored consumer expectations for all products and services, with reduced complexity, automated functionality, and application integration. There was a time when diabetic patients would need to manually draw out the correct insulin dosage from a vial and inject themselves with a syringe. This process required careful attention and could result in non-compliance with the required treatment schedule.
Physicians needed to provide significant training to ensure patients could maintain compliance. Fast forward 20 years, and numerous products are available, including the innovative continuous glucose monitoring (CGM) device and an external insulin pump.
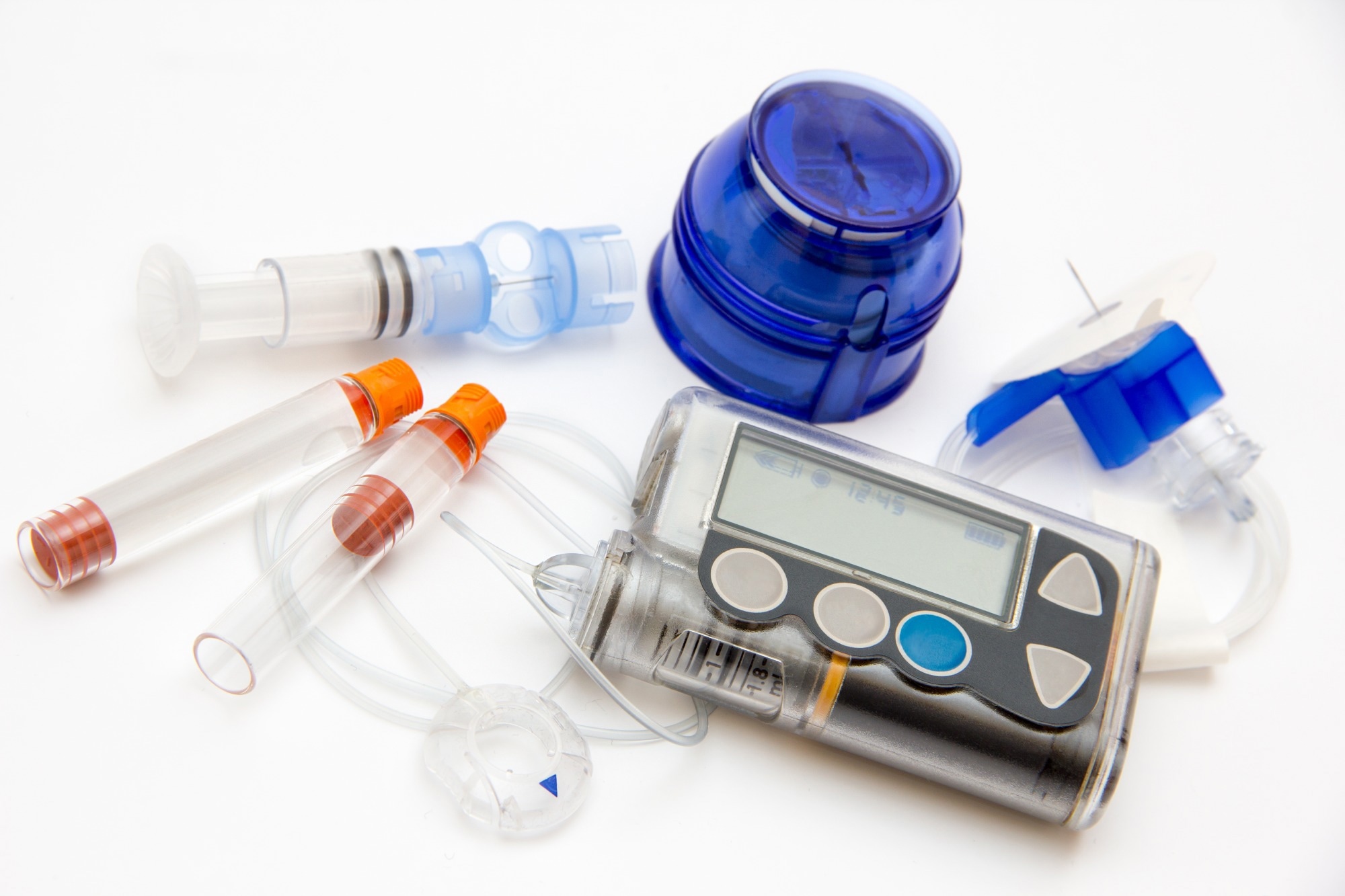
Image Credit: iStock
The CGM replaces finger pricking, providing real-time information on glucose levels in the patient, which is often tied to a software program. The programs have increased in sophistication and utilize advanced algorithms to learn a patient’s needs and predict when insulin may be required.
The insulin pump uses the glucose levels to adjust and deliver the correct amount of insulin. In some cases, there is no communication between the two, with a patient inputting the levels into the pump software. However, the two devices can more frequently communicate, taking the patient out of the process entirely.
Is this shift common for all diabetes patients?
A complete CGM-Insulin pump system is primarily required for patients with type 1 diabetes and more severe cases of type 2 diabetes. This solution provides a greater advantage for patients requiring basal and bolus insulin regimens.
Most type 2 diabetic patients use an insulin pen-style device because the dosing frequency may be much lower. The costs associated with insulin pens can be much less than insulin pumps. However, this may change as more healthcare programs cover insulin pumps and CGM devices.
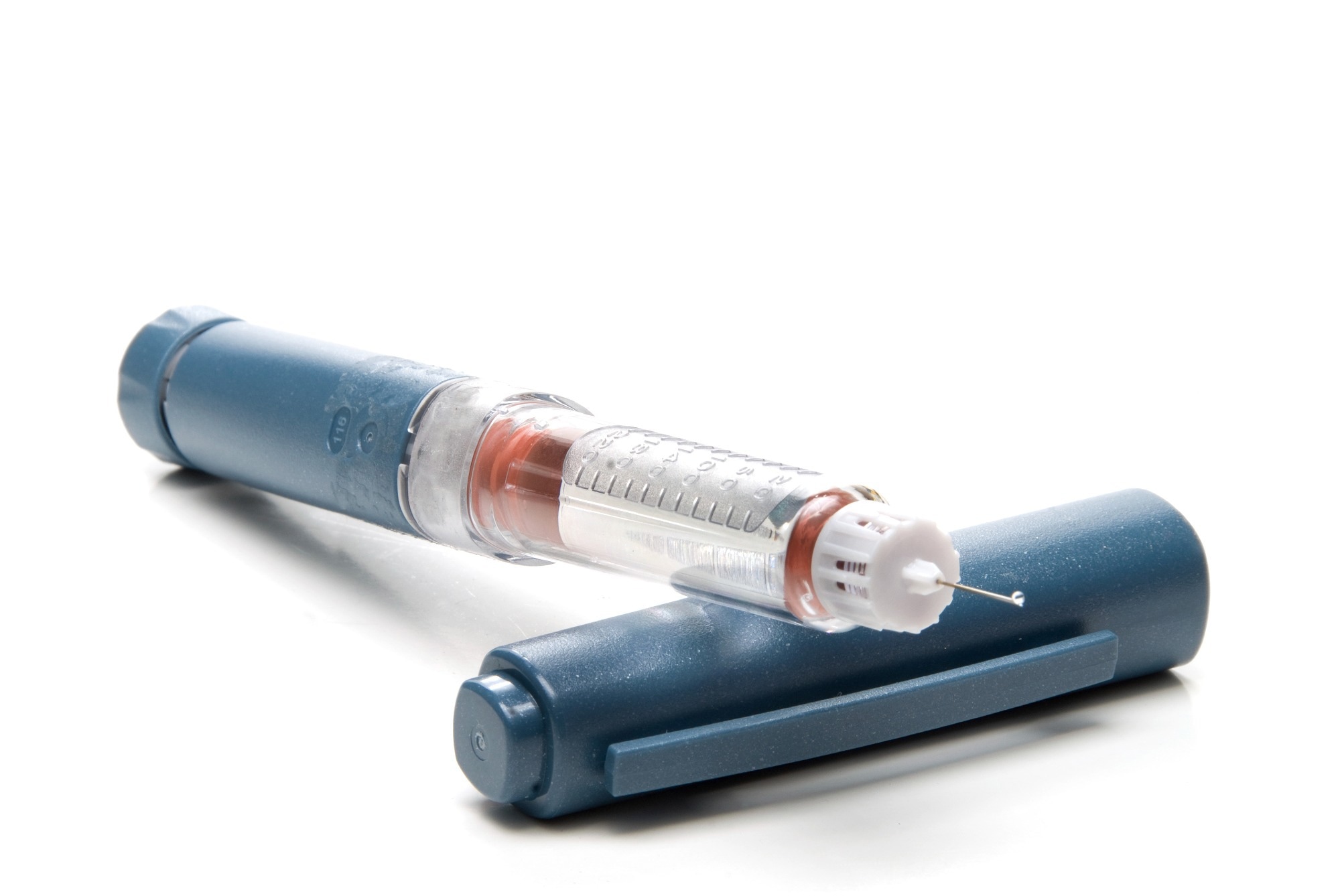
Image Credit: iStock
How has this led to the growth of mechanical testing in this area?
Anytime there is innovation within a field, testing requirements change to validate the usability and performance of new technology. The complexity of the device also invites additional means for testing. When looking at a prefilled syringe, mechanical testing defines the forces associated with actuating the syringe plunger.
As you move toward automated devices like an insulin pen, you must perform an additional layer of testing designed to ensure the automated functionality is doing what is expected.
This requires an expansion of capabilities typically associated with a mechanical test system. For example, it may be necessary to measure the delivered volume of the drug or identify auditory feedback to the patient, resulting in adding new measurements to the system.

Image Credit: Instron
The insulin pen is a familiar sight to many diabetes patients. How has mechanical testing shaped the development of this crucial device?
It is difficult to say whether the testing has shaped the ultimate design of the device. I would say that mechanical testing is the linchpin of the drug delivery design. The pharmaceutical manufacturer will provide a set of functional requirements for the device, often consisting of deliverable volume, delivery time, and storage conditions.
On the other end, a human factors team will create a list of requirements related to the device's usability, including forces required to activate the device or set dosage and any feedback given to the patient by the device. Mechanical testing is critical to proving the device meets functional and usability requirements.
What are some of the specific elements of this device that must be tested?
Insulin pens are typically variable-dose devices with interchangeable cartridges and screw-on needles. On the usability side, it is essential to understand the forces necessary to remove the cap and activate the device and the torques required to set the dose and attach/remove the needle.
The human factors study will provide information about the range of force/torque profiles for a representative sample of patients. Some devices will provide auditory feedback to minimize patient-device contact when the injection is complete. This feedback needs to be confirmed during the testing process.
To ensure functionality, the testing system will be required to evaluate the total delivered dose of the pen at different set volumes. If the device automatically injects insulin, it will be essential to capture the total delivery time as it can affect patient comfort.
When using an insulin pump, the delivery rate is even more critical as it will directly affect the insulin's efficacy. For insulin pumps or CGMs adhered to the patient’s body, understanding the adhesive profile is important to determine and guarantee the product will remain affixed during typical usage without resulting in Medical Adhesive Related Skin Injuries (MARSI).
Can you describe a mechanical testing system that might be needed to test specific elements of the insulin pen?
To test an insulin pen, the system must be capable of moving in both the linear and rotary axes while measuring force and torque. The system must also integrate additional devices such as a scale for measuring delivered volume or a microphone for auditory clicks.
To capture the complete volume of fluid, it is often necessary to provide a means for getting the last drop off the tip of the needle to be measured. In real-world usage, that is done automatically by removing the device from the patient, but on a test system, this can be managed by pressurized air focused on the needle tip.
Measurement of the delivery time can be done either by utilizing the scale data or through other sensors. The most commonly used are optical devices or laser-based technologies, which must also be integrated into the system.
What is the most complex aspect of this type of testing?
The most complicated part is creating a user interface for the system that can support these additional measurement transducers, making it simple for a device engineer to program the complete protocol.
The program must allow an engineer to sequence the interaction of the frame with the device and control all of the third-party sensors embedded in the system. Translating the complexity of the underlying control into an intuitive Human Machine Interface (HMI) is one of the most difficult aspects of mechanical system development and often the one that is most overlooked in a purchasing decision.
Can this testing be used to assess other drug-delivery devices?
An equivalent system can certainly evaluate a range of other drug-delivery devices. Autoinjectors used for other chronic diseases can also be evaluated on the system, as they have an overlapping set of requirements.
The main difference with autoinjectors is that they are more often single-use fixed dose devices where a pre-filled syringe is integrated into the device, rather than a cartridge. In this instance, an additional parameter must be monitored: the depth of the automatically actuated needle at the time of delivery to confirm the drug is reaching the therapeutically relevant region.
An advanced optical sensor can be added to evaluate the needle length and report it within the system software. These systems can also test simpler devices like pre-filled and safety syringes, only utilizing the force measurement capability.
Can you describe some machines Instron uses to mechanically test diabetes-related products?
Instron produces universal test systems, which means the underlying frame is designed to support a nearly endless range of applications. In the case of insulin pens and automatic drug delivery devices, the system has complex fixturing with sensitive measurement equipment, such as the attached scale. This equipment can be removed if necessary to allow for more standard testing.
Instron systems evaluate the attachable needles for insulin pens, determining whether the adhesion between the needle and the plastic hub it is housed in is sufficient. When examining the cartridges used in both pens and pumps, multiple functional tests can be conducted.
Residual Seal Force (RSF) testing may be performed on filled cartridges to ensure the sealing process was completed to specification and there is no risk of contamination. The cartridge may also undergo Break Loose Glide Force (BLGF) testing to evaluate the interaction between the coating properties of the inner wall and the stopper. This testing is crucial in device development to understand the minimum forces required to deliver the drug.

Image Credit: Instron
What benefits does Instron mechanical testing present to the end user?
The goal is to produce traceable data to validate that a device can meet regulatory requirements and provide an acceptable user experience. FDA submissions require excessive amounts of data related to design verification, performance data, and human factors studies.
A mechanical test system ensures quantitative analysis can be provided for the device throughout product development. A significant benefit is having a system combining multiple tests into a single process, reducing total test time and material needed for evaluations.
This helps streamline the design verification process and speed up time to market while providing the design team with accurate feedback on performance. An Instron system provides the benefit of toeing the line between creating an extremely flexible system capable of supporting R&D activities while offering robust product and automation capabilities necessary for the rigor of production.
About the speaker

Landon Goldfarb is the Biomedical Market Manager at Instron where he works with the world's leading biomedical companies to implement, improve, expand, and automate their static mechanical testing.

This information has been sourced, reviewed and adapted from materials provided by Instron.
For more information on this source, please visit Instron.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.