Sponsored by HORIBAReviewed by Olivia FrostJan 15 2026
Plastic pollution is a major global problem, harming ecosystems at every level of the food web. Discarded fishing gear is a leading source, making up around 85 % of the plastic waste found in marine environments.1

Image Credit: Zephyr_p/Shutterstock.com
Nylon fishing lines and nets are particularly durable plastics, with lifespans extending into the hundreds of years, causing significant issues to marine life.2
To tackle the issue, organisations like Net Your Problem collect damaged or abandoned fishing nets, sort and organise them, and send them to recycling facilities where they are processed into pellets for commercial use.3
An important aspect of this process is the accurate identification of the polymer composition of the fishing nets to ensure proper sorting into the appropriate recycling streams, such as nylon, polypropylene, and polyethylene.
It can be particularly challenging to do so due to the vast amount of different nylons used in fishing gear. Unambiguously determining the exact type of nylon is a critical step in the sorting process.
Raman spectroscopy has proven to be an effective technique for distinguishing between various types of polymers and within polymer classes, such as nylon.
Nylon has unique characteristics in Raman spectra, which allow for its definitive identification without the need for a high-resolution Raman microscope.
Figure 1 displays spectra of reference nylon materials recorded using HORIBA’s MacroRAMTM benchtop Raman spectrometer.
Each polymer presents clearly distinguishable spectral fingerprints that facilitate the identification of unknown samples, including abandoned fishing gear.
HORIBA investigated four monofilament gillnets and one polytwine gillnet, analyzing the samples using Raman spectroscopy (see Figure 2). The samples were collected by the Cape Cod Commercial Fisherman’s Alliance and originated from various states in southern New England, including Massachusetts, New Hampshire, and Rhode Island.
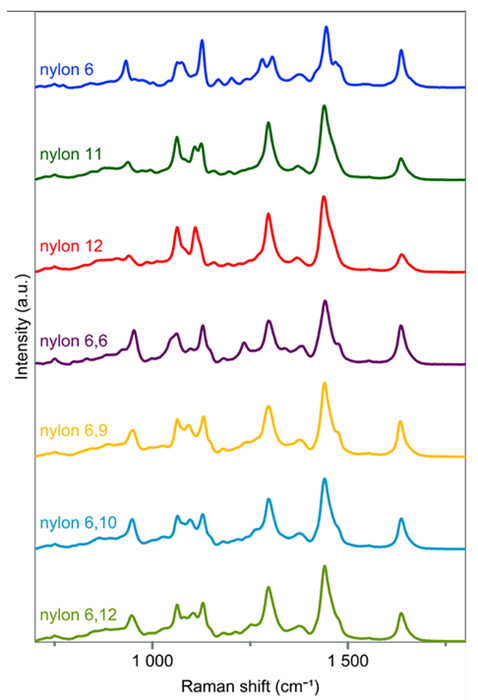
Figure 1. Raman spectra of nylon 6, nylon 11, nylon 12, nylon 6,6, nylon 6,9, nylon 6,10 and nylon 6,12 recorded with HORIBA’s MacroRAMTM benchtop Raman spectrometer. Reference nylon materials were sourced from PolySciences, Inc. Image Credit: HORIBA
HORIBA’s MacroRAMTM benchtop Raman spectrometer was paired with a BallProbe® (1/8”, MarqMetrix®) to enable rapid, alignment-free Raman characterization and identification of unknown polymer types.
Near-infrared 785 nm laser excitation was used to suppress fluorescence induced by the pigmented fibers (white, red, green, and blue).
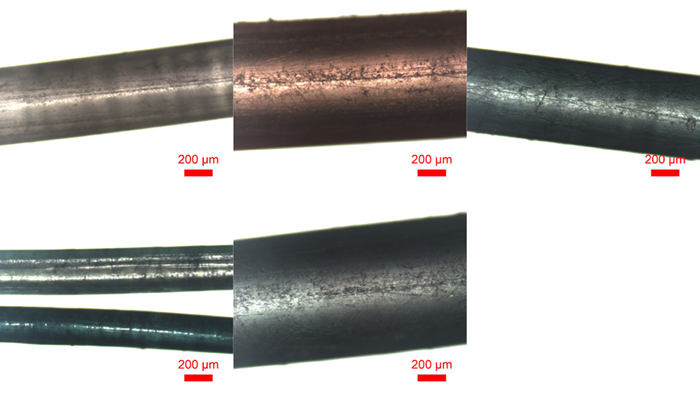
Figure 2. Optical micrographs recorded of various fiber samples; white monofilament gillnet, red monofilament gillnet, green monofilament gillnet, green polytwine gillnet, and blue monofilament gillnet (from top left to bottom right). Image Credit: HORIBA
The spectra recorded from the monofilament samples indicate that all samples are composed of the same variety of nylon. A close comparison of the unknown spectra with the nylon reference material spectra revealed that all monofilament samples can be classified as nylon 6.
Figure 3 presents the processed spectra of each unknown sample after baseline subtraction alongside the reference spectra of nylon 6 (top) and nylon 6,6 (bottom). The doublet at approximately 1300 cm-1 clearly indicates the assignment of nylon 6 over other varieties.
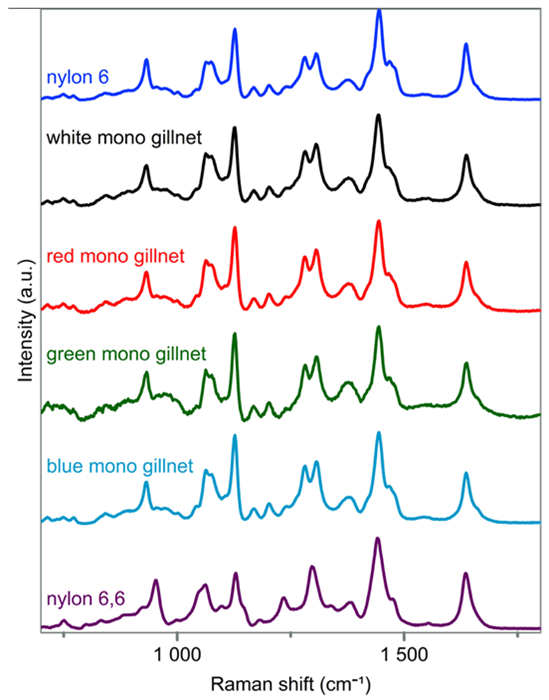
Figure 3. Reference spectra of nylon 6 and nylon 6,6 are shown at the top and bottom, respectively. The spectra of unknown monofilament gillnet samples are plotted in the middle labeled by color (white, red, green, blue). Image Credit: HORIBA
The analysis of the green polytwine gillnet sample was more complicated. Polytwine gillnets consist of multiple filaments interlaced to form a braid, which make it harder to analyze.
When the braid was unraveled, it revealed two distinct filament types making up the polytwine: a lighter, slightly thicker set at the centre and a darker, thinner set around the edges.
A small sample from each type of filament was trimmed for analysis. Raman spectroscopy showed that both types exhibited the same spectral bands. However, there was a notable difference in the relative intensities of the Raman bands for the lighter and darker strands, as illustrated in Figure 4.
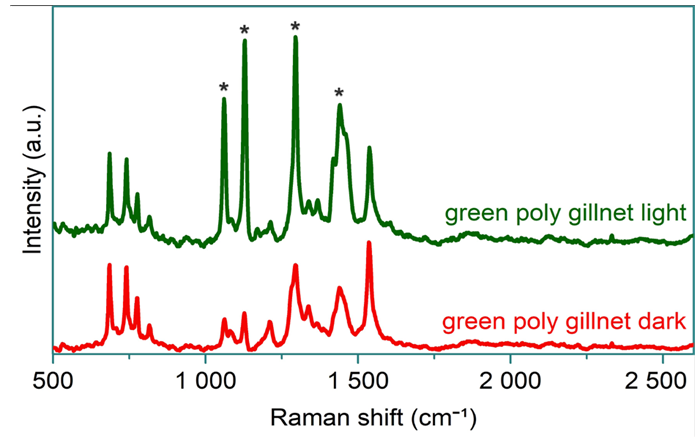
Figure 4. Baseline corrected spectra of light (top) and dark (bottom) colored strands from the green polytwine gillnet sample. The asterisks indicate bands of significantly differing intensity between the two measurements. Image Credit: HORIBA
Using HORIBA’s LabSpec 6 software suite, the spectrum obtained from the darker colored strand was subtracted from that of the lighter colored strand, and vice versa. The resultant spectra closely aligned with polyethylene (light–dark) and pigmosol green (dark–light), as illustrated in Figure 5 and Figure 6, respectively.
Analysis of this unknown sample indicated that the polytwine gillnet was polyethylene rather than nylon. Despite significant spectral contributions from the pigment, the polymer was confidently identified.
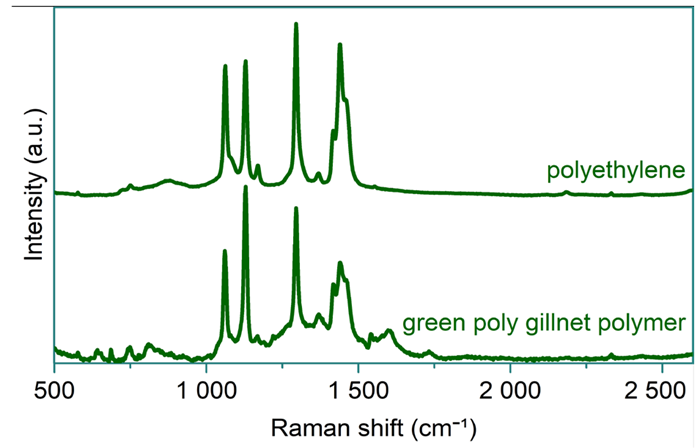
Figure 5. Reference spectrum of polyethylene (top) and subtracted spectrum of the light strand from the green polytwine gillnet sample (bottom). Image Credit: HORIBA
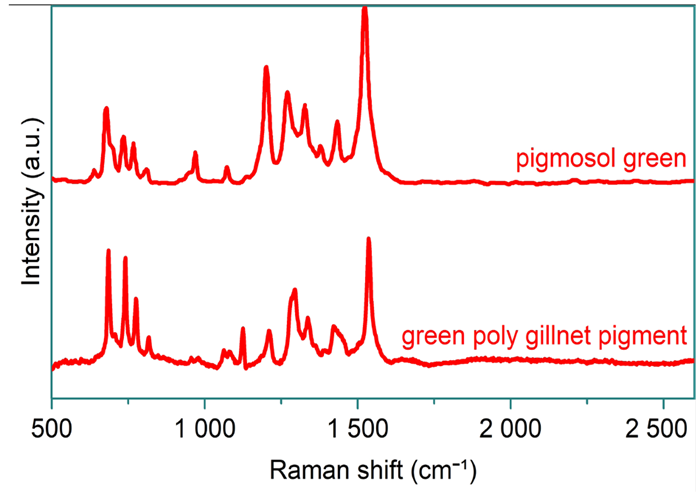
Figure 6. Reference spectrum of pigmosol green from Wiley’s KnowItAll software and spectral libraries (top) and subtracted spectrum of the dark strand from the green polytwine gillnet sample (bottom). Image Credit: HORIBA
Although these measurements were conducted in a laboratory setting, the results indicate that the MacroRAMTM could be effectively deployed for field measurements at the site of abandoned nets.
Identification can be performed quickly and efficiently in real time while sorting through fishing gear and preparing it for shipment, using a remote touch probe.
Furthermore, LabSpec 6's advanced data processing capabilities facilitate more complex post-acquisition analyses that are unattainable with simpler handheld Raman devices.
This is particularly advantageous for samples with substantial spectral contributions from pigments, allowing for unambiguous identification of the polymer type.
These findings are promising for measuring microplastics in a rapid, alignment-free way. The unknown fiber samples analyzed in this study ranged from 260 μm to 920 μm in diameter, well within the definition of microplastics (< 5 mm). Microplastic samples larger than 100 μm are routinely hand-picked for observation using a stereo microscope.
Integrating a benchtop Raman system with a remote touch probe enables rapid chemical identification at the point of microplastics sorting and classification based on size, morphology, and color.
Future advancements could involve deploying the unit in the field to measure microplastics at their source, such as along coastlines or on boats.
In conclusion, the MacroRAMTM benchtop Raman spectrometer, when used with a remote touch probe, accurately and efficiently identifies polymer types from various colored fishing nets.
There is a clear distinction among different polymer classes (polyethylene and nylon) as well as within a single polymer class (nylon 6 and nylon 6,6).
The methods demonstrated here can be easily adapted for field measurements at abandoned fishing gear sites or for monitoring microplastics at their source.
Acknowledgements
Produced from material originally authored by Bridget O’Donnell from HORIBA Instruments.
References and Further Reading
- Greenpeace International. (2019). Greenpeace International. (online) Available at: https://www.greenpeace.org/international/publication/25438/ghost-gear/.
- US Department of Commerce, N.O. and A.A. Can marine debris degrade on its own in the environment? (online) National Oceanic and Atmospheric Administration (NOAA). Available at: https://oceanservice.noaa.gov/facts/degrade.html.
- Net Your Problem. (2025). Net Your Problem - Dev. (online) Available at: https://www.netyourproblem.com (Accessed 18 Jul. 2025).

This information has been sourced, reviewed and adapted from materials provided by HORIBA.
For more information on this source, please visit HORIBA.