Dec 22 2014
Princeton University researchers have used theoretical calculations to report novel insights into the structure of an active component of the nickel oxide catalyst, called â-NiOOH. Princeton Professor of chemistry Annabella Selloni published the findings in The Journal of Physical Chemistry Letters on October 28, 2014.
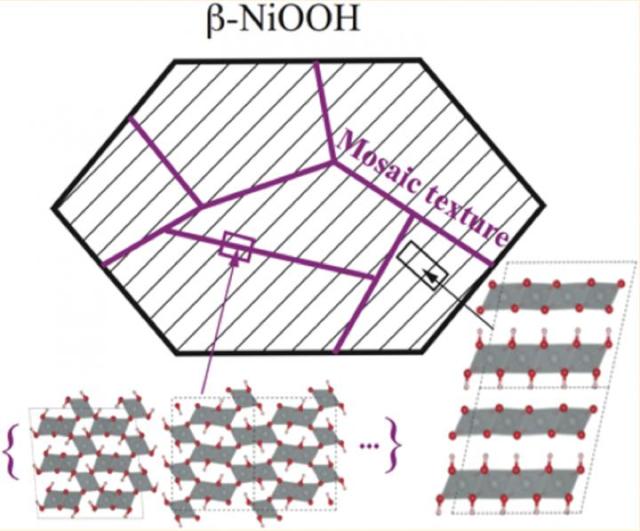 This image is a representation of the mosaic structure of â-NiOOH and its possible structures. Credit: Selloni lab
This image is a representation of the mosaic structure of â-NiOOH and its possible structures. Credit: Selloni lab
A potential clean energy source is hydrogen fuel, which can be obtained by splitting water into oxygen and hydrogen. It is possible to perform this reaction by using a catalyst, which helps to speed up any reaction. However, currently used catalysts do not have the efficiency needed to make this process commercially viable.
Scientists have recently discovered the catalyst of nickel oxide doped with iron, which is a highly active compound that can accelerate this reaction. However it is not yet fully understood from where its activity originates.
"Understanding the structure is the basis for any further study of the material's properties. If you don't know the material's structure you can't know what it's doing," Selloni said.
It has been tough to determine experimentally the exact structure of nickel oxide as it constantly changes during the reaction.
The research team adopted a theoretical approach and used a "genetic algorithm" for searching the structure. Genetic algorithms work under a specific set of parameters, which obtain inspiration by creating several generations of structures in order to attain the most likely or most “fit” candidates.
Li and Selloni considered the results obtained from the genetic algorithm search along with computational techniques such as hybrid density functional theory calculations that estimate the electronic structure of a molecule in order to determine nickel oxide structures that supported existing observations.
The material’s mosaic texture, which includes grain-like microstructures is one such observation. According to the researchers, these microstructures are stable tunnel structures, which relieve the stress between layers. Another feature observed is distance doubling between layers of the same material, which is referred to as its c axis periodicity, representing alternating Ni(OH)2 and NiO2 layers formed during the reaction.
Having a better understanding of the structure of the material, the scientists can further map its activity in the reaction. "I'm interested in the microscopic mechanisms, what are the electrons and atoms doing?" Selloni said.