Jul 13 2015
Researchers at the Department of Energy's SLAC National Accelerator Laboratory have tested a new material design that enhances the ability of inexpensive solar panels to collect solar energy and release it in the form of electricity.
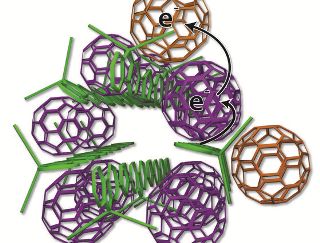 Scientists devised a new arrangement of solar cell ingredients, with bundles of polymer charge donors (green rods) and neatly organized spherical carbon molecules, also known as fullerenes or buckyballs, serving as charge acceptors (purple, tan). The researchers studied the new design at SLAC's Stanford Synchrotron Radiation Lightsource. (UCLA)
Scientists devised a new arrangement of solar cell ingredients, with bundles of polymer charge donors (green rods) and neatly organized spherical carbon molecules, also known as fullerenes or buckyballs, serving as charge acceptors (purple, tan). The researchers studied the new design at SLAC's Stanford Synchrotron Radiation Lightsource. (UCLA)
Scientists at the University of California, Los Angeles (UCLA) discovered that when they assembled solar panel components in a manner that was similar to the way in which plants harvested energy, it is possible to split the negative and positive charges in a stable manner for time periods lasting up to several weeks. Existing solar panels are capable of storing these charges just for millionths of a second.
“In photosynthesis, plants that are exposed to sunlight use carefully organized nanoscale structures within their cells to rapidly separate charges – pulling electrons away from the positively charged molecule that is left behind, and keeping positive and negative charges separated,” said Sarah Tolbert, a UCLA professor of chemistry and one of the senior authors of the research. “That separation is the key to making the process so efficient.”
The X-ray studies required for this research were conducted at SLAC's Stanford Synchrotron Radiation Lightsource (SSRL). These studies enabled the researchers to microscopically view various material designs and ascertain the optimum nanoscale structure that was best for separation of charges.
Silicon is used in traditional rooftop solar cells for capturing solar energy. While these are expensive, plastics and other materials that are comparatively lower in cost are also used. However, plastic cells demonstrate comparatively less efficiency. This is due to the fact that recombination occurs before the positive and negative charges (that are in a separate state in the material) can become electrical energy.
“Modern plastic solar cells don’t have well-defined structures like plants do because we never knew how to make them before,” Tolbert said. “But this new system pulls charges apart and keeps them separated for days, or even weeks. Once you make the right structure, you can vastly improve the retention of energy.”
The system developed by the UCLA researchers is made up of polymer strands, which form the building block of plastics. Sunlight is absorbed by these strands and the electrons pass through to a fullerene. Fullerenes or 'buckyballs' are spherical carbon molecules.
In these solar cells, the materials are organized in a manner that resemble cooked pasta. The long polymers resemble disorganized spaghetti, while fullerenes resemble meatballs. However, current cannot be easily extracted from this cell due to this arrangement. Sometimes, electrons get lost when they jump back into the polymer spaghetti.
A neater method for arranging the elements was worked out by the researchers. Meatballs are placed in precise locations in small uncooked spaghetti bundles. Some fullerenes are made to sit outside the polymer spaghetti bundles, while the others fullerenes are made to sit inside.
The electrons in the polymers are taken by the fullerenes inside the structure and tossed to the fullerenes on the outer side. This would allow the electrons to be kept apart from the polymer for time durations lasting weeks. The optimum arrangement for the buckyballs and the polymer strands were obtained after numerous experiments were conducted at SSRL and by other resarchers.
“When the charges never come back together, it becomes easier to get them out of the solar cell in the form of electricity,” said Benjamin Schwartz, a UCLA professor of chemistry and a co-author of the study. “This is the first time such long charge lifetimes have been shown using this type of material.”
The team observed that when placed closely together the materials assembled by themselves into this specific form. When compared to currently used technologies, the new design was more eco-friendly. The technologies that are currently used employ toxic organic solutions, while in the new method the materials assemble in water.
“Once you make the materials, you can dump them into water and they assemble into the appropriate structure because of the way the materials are designed,” Schwartz said.
Tolbert states that follow-up research is being planned at SSRL, and further research in being conducted to determine the manner in which this new technology can be incorporated into real solar cells.
The study results have been published in the journal Science.