Reviewed by Lexie CornerJun 20 2025
Professor Ji-Sang Yu and Professor Hyun-seung Kim of the Korea Electronics Technology Institute (KETI) and Kangwon National University in South Korea recently published a study in Nano-Micro Letters. The study explored a new approach to addressing these issues by adding lithium salt (LiPF6) to the electrolyte.
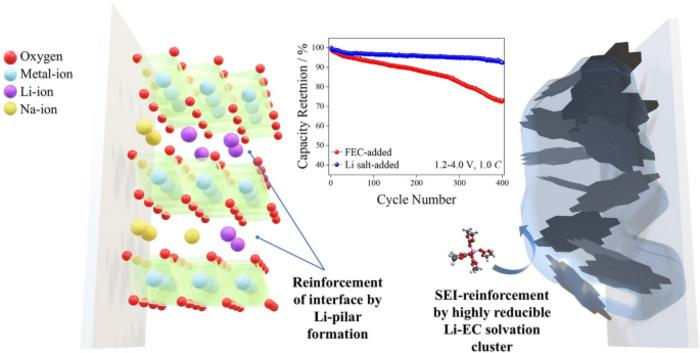 LiPF6 integration into sodium-ion battery electrolytes strengthens solid electrolyte interphase (SEI) film and stabilizes O3 electrode surfaces, enhancing cycleability with 92.7 % at 400 cycles. Li-based SEI exhibits reduced solubility, effectively suppressing sodium-ion and electron leakage, and mitigating electrolyte decomposition on hard carbon electrode. The formation of Li-ion pillars on O3-type electrode surfaces significantly reduces oxygen release and electrolyte degradation, resulting in improved capacity retention. Image Credit: Jooeun Byun, Joon Ha Chang, Chihyun Hwang, Chae Rim Lee, Miseung Kim, Jun Ho Song, Boseong Heo, Sunghun Choi, Jong Hyeok Han, Hee-Jae Jeon, Beom Tak Na, Youngjin Kim, Ji-Sang Yu, Hyun-seung Kim.
LiPF6 integration into sodium-ion battery electrolytes strengthens solid electrolyte interphase (SEI) film and stabilizes O3 electrode surfaces, enhancing cycleability with 92.7 % at 400 cycles. Li-based SEI exhibits reduced solubility, effectively suppressing sodium-ion and electron leakage, and mitigating electrolyte decomposition on hard carbon electrode. The formation of Li-ion pillars on O3-type electrode surfaces significantly reduces oxygen release and electrolyte degradation, resulting in improved capacity retention. Image Credit: Jooeun Byun, Joon Ha Chang, Chihyun Hwang, Chae Rim Lee, Miseung Kim, Jun Ho Song, Boseong Heo, Sunghun Choi, Jong Hyeok Han, Hee-Jae Jeon, Beom Tak Na, Youngjin Kim, Ji-Sang Yu, Hyun-seung Kim.
Sodium-ion batteries (SIBs) are being explored as a potential alternative to lithium-ion batteries. This is due to the wide availability of sodium and the ability of SIBs to store large amounts of energy at a lower cost.
However, their commercialization faces challenges. These include low cycle stability and significant capacity loss, mainly caused by a weak solid electrolyte interphase (SEI) and interface degradation.
Why Lithium Salt Matters
Improved SEI Formation:
Adding LiPF6 to the electrolyte helps form a SEI layer on the hard carbon anode. This SEI is more stable and less soluble than the sodium-based SEI. It reduces sodium-ion and electron leakage and slows down electrolyte decomposition.
Surface Stabilization of O3-Type Cathode:
LiPF6 also leads to slight surface doping of the O3-type cathode. This introduces lithium ions that act as structural pillars. These pillars help reduce oxygen release and electrolyte breakdown, improving capacity retention and cycle stability.
Innovative Design and Mechanisms
Modified Solvation Structure:
LiPF6 changes how ions are surrounded by solvent molecules in the electrolyte. It creates a more easily reduced lithium-solvated cluster compared to a sodium-solvated one. This helps form a stable, protective SEI layer on the hard carbon anode.
Formation of Li-ion Pillars:
The small amount of lithium ions introduced into the O3-type cathode acts like pillars. These pillars support the cathode’s layered structure, reducing structural collapse and gas release during charge/discharge cycles.
Results and Discussion
Improved Cycle Performance:
Adding LiPF6 led to improved cycling stability. The batteries retained 92.7 % of their capacity after 400 cycles, higher than the typical 80 % retention in similar sodium-ion batteries.
Reduced Gas Evolution:
Measurements using differential electrochemical mass spectrometry (DEMS) showed less CO2 released from the cathode surface. This indicates reduced oxygen loss and less electrolyte degradation.
Enhanced Interfacial Stability:
Post-experiment analysis using high-resolution transmission electron microscopy (HR-TEM) and X-ray photoelectron spectroscopy (XPS) showed a stable SEI on the hard carbon anode and a preserved structure in the O3-type cathode.
Future Outlook
Scalability and Practical Applications:
The use of LiPF6 in the electrolyte can be scaled for larger production. These findings could support the development of more cost-effective and efficient sodium-ion batteries for real-world use.
Future Research:
Future studies may explore other additives or changes to the electrolyte to improve battery performance and stability. Advanced characterization techniques could help reveal more details about how interfaces behave during operation.
Conclusion
This study shows a new way to improve sodium-ion batteries by adding lithium salt to the electrolyte. A stronger SEI layer and a more stable cathode structure lead to better performance and longer cycle life.
These results support the development of affordable, high-performance sodium-ion batteries, which could contribute to a more sustainable energy future.
Journal Reference:
Byun, J., et al. (2025) Transformative Effect of Li Salt for Proactively Mitigating Interfacial Side Reactions in Sodium-Ion Batteries. Nano-Micro Letters. doi.org/10.1007/s40820-025-01742-z.