Researchers at the Catalan Institute of Nanoscience and Nanotechnology (ICN2) have developed a pioneering method for obtaining various types of organic molecule through a process of molecular 'cutting'. This approach allows the rapid and precise isolation of target molecules, avoiding the slower and more complicated procedures usually associated with traditional chemical synthesis. These results pave the way for the easier and more efficient production of complex molecules, with promising applications in areas such as the development of new materials.
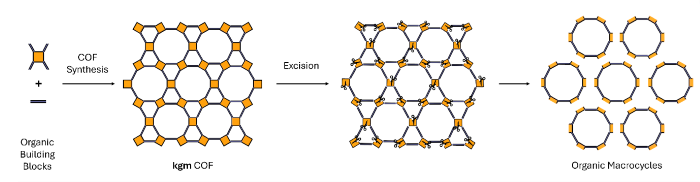 Diagram of the synthesis of COFs and the subsequent release of macrocycles through ozonolysis. Image Credit: ICN2
Diagram of the synthesis of COFs and the subsequent release of macrocycles through ozonolysis. Image Credit: ICN2
Specifically, the study introduces a method of extracting macrocycles, which are cyclic organic molecules that are already used in several industries, such as food, cosmetics, and drug delivery. To obtain them, the team started with larger porous crystalline structures called COFs (covalent organic frameworks), which contained the desired macrocycles within their molecular framework. COFs are widely studied molecules due to their potential applications in areas such as gas storage.
The work, published in the journal Science, is the result of an international collaboration involving researchers from several institutions, including the Universitat Autònoma de Barcelona (UAB), the University of Girona, the University of California Berkeley, and the Institute of Materials Science of Barcelona (ICMAB-CSIC).
Clip-off Chemistry: The Art of “Cutting Out” Molecules
The technique is based on the concept of Clip-off Chemistry, developed under the leadership of ICREA Prof. Daniel Maspoch, head of the ICN2 Supramolecular NanoChemistry and Materials Group and lead author of the study. The work was also led by other members of his group, Drs. Inhar Imaz and Jorge Albalad. This strategy relies on materials that already incorporate the target molecules within their structure, which are then selectively "clipped off" and released. The COFs used in the study were designed in advance using simple molecular precursors, with specific chemical bonds incorporated at strategic positions, such as double and triple bonds between carbon atoms which can be selectively broken.
Once the COFs had been synthesized, the next step was to release the macrocycles. To achieve this, the researchers employed ozone gas as a molecular 'scalpel'. This gas is made up of three oxygen atoms and can break these reactive bonds through a process known as ozonolysis. This releases the macrocycles quickly and efficiently, eliminating the need for slow and complex synthesis routes.
As Prof. Maspoch explains: 'We design materials that already contain the rings we’re aiming for, using simple building blocks — like LEGO pieces — and then we release them with surgical precision.'
Enormous Potential Across Fields
Using this strategy, the researchers successfully synthesized nine different types of macrocycle, some of which contained up to 162 atoms. These included several chemical structures and various functional groups, such as aldehydes and carboxylic acids, which demonstrates the technique's versatility.
The resulting macrocycles' structure was confirmed using advanced techniques such as mass spectrometry and scanning tunnelling microscopy. Significant contributions to these analyses were made by the ICN2 Atomic Manipulation and Spectroscopy Group, led by ICREA Prof. Aitor Mugarza.
According to the authors: 'This method paves the way for a new and versatile approach to obtaining complex molecules. It has enormous potential for use in diverse fields such as organic chemistry, nanotechnology, and the development of new materials, devices, and biosensors.”