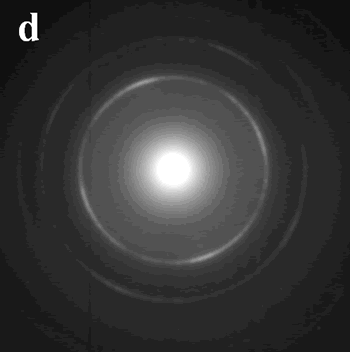
Introduction During the last two decades, there has been an enormous interest in nanostructures due to their conspicuous physical-chemical properties that differ markedly from those of bulk materials[1]. Various methods, such as hydrothermal and solvothermal routes[2], surfactant-assisted approach[3], have been utilized for the synthesis of nanomaterials. Most physical and chemical properties of these nanomaterials are sensitively dependent on their size and shape, so materials scientists are still focusing on developing simple and effective methods for the fabrication of nanomaterials of controlled size and morphology[4]. Since metal nanoparticles have various applications, the synthesis of metal nanoparticles has attracted much attention especially in the last decade[5]. A variety of techniques have been developed to synthesize metal nanoparticles, including chemical reduction using a number of chemical reductants including NaBH4, N2H4, NH2OH, ethanol, ethylene glycol and N,N-dimethyformamide (DMF)[6-10], aerosol technique[11], electrochemical or sonochemical deposition[12, 13], photochemical reduction[14], and laser irradiation technique[15]. Because of the size-dependent properties, many physical, chemical and electrochemical methods have been employed to get the metal nanoparticles with uniform size, such as NaBH4-reduction approach resulting in the thiol-capped 1.8-3.5 nm diameter silver nanoparticles and alcohol reduction of fatty acid silver salts under microwave irradiation[16, 17]. The assembly of uniform nanoparticles into well-defined two- and three-dimensional (2D and 3D) superlattices is critically important to chemical, optical, magnetic and electronic nanodevices and would bring possibilities to brand-new properties and applications that result from the spatial orientation and arrangement of the nanocrystals[18]. Therefore, several approaches, such as self-assembly[19], Langmuir-Blodgett (LB) techniques[7], and electrophoretic deposition method[20] have been used in order to obtain self-organized lattices of metal, oxide and chalcogenide nanoparticles including silver[11], gold[21], cobalt[22], indium[23], α-Fe2O3[24], cobalt oxide[25], BaTiO3[26], CdS[27], CdSe[28], and Ag2S[29] nanoparticle arrays. Besides the uniform and assembled nanoparticles, one-dimensional (1D) nanostructures, such as nanorods and nanowires, are also of particular interest not only because of their great potential for testing and understanding fundamental concepts but also because of their wide applications as interconnects in electronic devices with super-functions[30]. The synthesis of 1D nanostructures and guiding these nanometer-scaled building blocks to ordered superstructures would offer great opportunities to investigate the size- and dimensionality-dependent properties of these materials and could lead to the construction of nanoscale devices[31]. Until now, great progress has been made in the shape control of nanomaterials and a range of different 1D nanostructures have been fabricated by various techniques, such as Vapor-Liquid-Solid (VLS) growth mechanism[32], micro-emulsion method[3], hydrothermal (or solvothermal) technique[2] and template methods[33]. Among the various methods, hard template method is an effective method to obtain the nanostructures with low dimensionality. Porous alumina membrane and mesoporous materials such as SBA-15 are two of the most used templates. Nanowires of Ag, Pt, and Au were grown in the nanochannels of SBA-15[34], and many other nanorods arrays have been obtained by porous alumina membrane[35]. However, the pore size of the alumina membrane is from dozens of nanometers to several hundred nanometers and the SBA-15 is usually in powder form or as membranes with its channels parallel to plane of the substrate, which limits their applications in nanodevice fabrication[36]. Combining these two templates by introducing SBA-15 into alumina membrane channels is expected to find super-function in nanowire fabrication and bio-molecule separations. This review paper shows that conventional and microwave-assisted hydro- or solvothermal methods are highly suited for the synthesis of nanomaterials of controlled size and shape under environmentally benign conditions for several different applications. Experimental Microwave-Assisted Solvothermal Synthesis of Metal Nanoparticles For the hexagonally arranged spherical silver nanoparticles, 0.15 g AgNO3 was added in a Teflon vessel of a double-walled digestion vessel used in MARS-5 system. Then 10 ml toluene, 1 ml dodecylthiol and 4 ml ethylene glycol were added into the vessel in order. After sealing, the vessel was treated at 160°C for 3 hours using a microwave digestion system, MARS-5 (CEM Corp.). After cooling to room temperature, the product was collected and an interface between two layers was found to be full of black product. Pt and Pd nanoparticles were synthesized by microwave-assisted solvothermal method. PVP with an average molecular weight of 40K was used as a capping agent in all the experiments. Dihydrogen hexachloroplatinate (IV), and palladium (II) 2,4-pentanedionate were used as metal precursors. PVP was dissolved in methanol or ethanol and then the metal salts were added. The reactants were heated for 60 min at 90°C when methanol was used as a reducing agent and at 120°C when ethanol was used as a reducing agent for 60 min under microwave irradiation. Biomolecule-Assisted Hydrothermal Synthesis For Te Nanowires[37] For the elemental tellurium nanowires, 0.15g H2TeO4·2H2O was mixed with 0.075 g alginic acid in 10 ml distilled water in a Telfon-lined stainless autoclave. After sealing, the autoclave was heated to 150°C and kept for 15 hours. After cooling to room temperature, the solid product was collected by centrifugation at 2000 rpm for ~10 min and washed with distilled water and alcohol several times, followed by drying in air at room temperature. Sol-Gel Method for The Growth of SBA-15 Nanorods Array Inside Porous Alumina Membrane[38] In the synthesis of SBA-15 nanowire arrays inside porous alumina membrane, a sol solution was made by dissolving 1 g Pluronic P123 (PEO20PO70EO20, Mav=5800, Aldrich) in 5 g ethanol and 0.2 g 2 M HCl solution and mixing with 2.08 g tetraethyl orthosilicate (TEOS, 98%, Aldrich). Then a simple piece of porous alumina membrane was put into the sol solution. After the sol solution was left at room temperature (about 25°C) for 20 h to make sol change to gel, some amount of liquid paraffin wax with thickness of 1 mm was poured onto the gel and then it was kept at 60°C for 20 h. Then, liquid paraffin was removed and the sample was calcined in the alumina membrane at 540°C for 6 h. Nanowires of Pt Inside SBA-15 First, SBA-15 was prepared and treated with H2PtCl6 followed by H2 reduction at 400°C in order to prepare nanowires of Pt inside SBA-15. The SBA-15 was then dissolved in dilute HF solution to recover Pt nanowries. Characterization The morphology, crystallinity, and size of products have been determined by transmission electron microscopy (TEM) and scanning electron microscopy (SEM). Selected area electron diffraction (SAED) was used to identify the crystalline phases. TEM was carried out with a Philips 420 transmission electron microscope operated at 120 kV and SEM was carried out with a Hitachi S-3500N scanning electron microscope. Results and Discussion Microwave-Assisted Solvothermal Synthesis of Metal Nanoparticles Due to the spatial orientation and arrangement of the nanocrystals, 2D and/or 3D nanoparticles superlattices would bring possibilities to brand-new properties and applications, which make their syntheses be a focusing area in the current research field [18-20]. For the assembly of uniform Ag nanocrystals, the presynthesis of uniform nanoparticles or precursors is usually required followed by the organization process by surfactants or ligands. The development of a simple and direct method for the fabrication of such crystals is a major challenge for future research. Herein we report a general and one-step microwave-assisted interface-reaction for the synthesis and assembly of monodispersed silver nanoparticles. By using dodecylthiol as directing reagent and ethylene glycol as reducing agent, hexagonally arranged spherical silver nanoparticles can be obtained by a one-step interface-reaction under microwave-assisted solvothermal conditions without the requirement of the pre-synthesis of uniform silver nanoparticles or special precursors and the technique of size-selective precipitation. In the synthetic system, ethylene glycol and toluene form two layers with an interface where the thiol group of dodecylthiol might react with silver ions to form an inorganic-organic complex, which is reduced to elemental silver by ethylene glycol under microwave-solvothermal conditions. After the reaction, a black thin layer of silver nanoparticles is found at the interface and the formed silver nanoparticles automatically compact-pack to form ordered superstructures. Figure 1 shows the TEM images of the as-prepared sample, from which it can be clearly seen that the sample consists of a hexagonal-like ordered superstructure of monodispersed silver nanoparticles. Figure 1a displays a TEM image with low magnification, clearly showing that the two-dimensional (2D) hexagonal superlattice is the typical structure of the as-prepared silver sample. A TEM image of the silver sample with high magnification (Figure 1b) displays clearly that these nanoparticles are monodispersed with an average diameter of ~ 10 nm and the inter-particle spacings are calculated to be about 2 nm. Figure 1c shows its Fourier transform power spectrum. It displays ordered hexagonal-like spot arrays, which confirms the formation of the hexagonally arranged silver superlattice. The SAED pattern of the sample, showed in Figure 1d, exhibits polycrystalline diffraction rings, which can be indexed as cubic-phase metal silver, indicating that these nanoparticles are crystalline metal silver. | 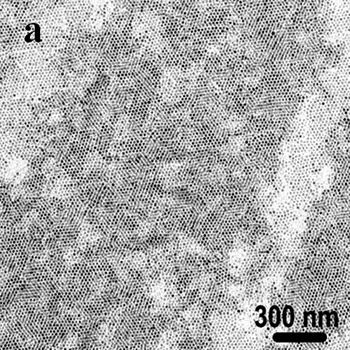


| | Figure 1. TEM images, Fourier transform power spectrum and SAED pattern of the synthesized silver sample under microwave-assisted solvothermal conditions. | Figure 2 shows the TEM images of synthesized Pt and Pd nanoparticles with methanol or ethanol as reducing agents. Using methanol as reducing agent, Pt nanoparticles were synthesized. Figure 2a shows the morphology of Pt nanoparticles formed with the PVP to Pt(IV) ratio of 18 and the concentration of Pt(IV) at 0.9 mM at 90°C. The particle size is about 3 nm. Pd nanoparticles can be also synthesized by using methanol as reducing agent. Figure 2b shows the TEM image of Pd nanoparticles formed at 90°C with the PVP to Pd(II) ratio of 1.8 and the concentration of Pd(II) at 9 mM and the particle size is about 10 nm. Using ethanol as reducing agent, Pt nanoparticles of approximately 3 nm were also obtained. Figure 2c shows the TEM image of Pt nanoparticles formed at 120°C with the PVP to Pt(IV) ratio of 18 and the concentration of Pt(IV) at 9 mM. Pd nanoparticles of around 10 nm were also synthesized by using ethanol as reducing agent. Figure 2d shows TEM image of Pd nanoparticles formed at 120°C with the PVP to Pd(II) ratio of 18 and the concentration of Pd(II) at 9 mM. Thus, Pt and Pd nanoparticles were successfully synthesized using either methanol or ethanol as reducing agents with microwave-assisted solvothermal technique. Synthesized Pt nanopartices are about 3 nm and Pd nanoparticles are around 10 nm. 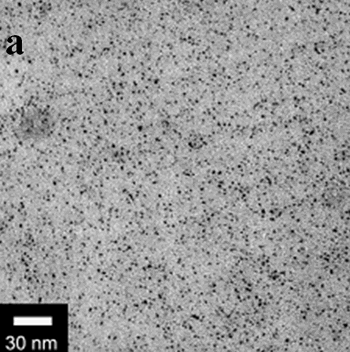
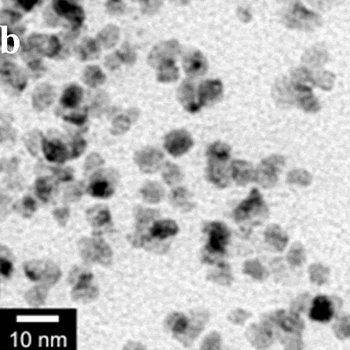
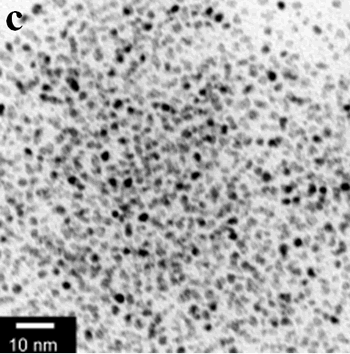
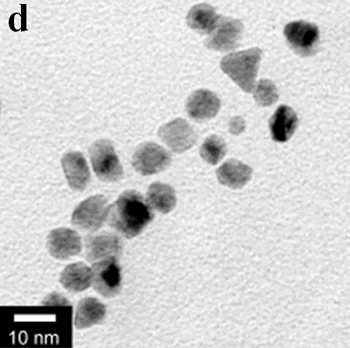
Figure 2. TEM images of the obtained Pt and Pd nanoparticles under microwave-assisted solvothermal conditions. Biomolecule-Assisted Hydrothermal Synthesis for Te Nanowires Bio-molecules, as life’s basic building blocks, have special structures with typical sizes in the range of about 5 to 200 nm, which is almost the same length scale as those of nanomaterials[39]. These biomolecules would be of great importance in developing novel materials and recently they have been introduced into the synthesis of nanomaterials[40, 41]. Alginic acid, a straight-chain polyuronic acid extracted from macrocystis pyrifera (kelp)[42], has been extensively used in pharmacy and cosmetic materials and recently as biosorption agent of heavy metals, which might be expected to be useful in controlled synthesis of nanomaterials. Elemental tellurium has a wide range of applications in various thermoelectronics, photoconductors and piezoelectronic devices and the availability of 1D Te nanostructure could bring forth new applications or enhance the performance of existing devices[43, 44]. Herein we report a mild bio-molecule-assisted hydrothermal method by using alginic acid as both reducing agent and directional template to obtain 1D Te nanowires from commercial H2TeO4 powders under conventional hydrothermal conditions. The as-synthesized nanowires’ structure and growth direction were characterized by TEM along with selected area electron diffraction (SAED) pattern. Figure 3 is the TEM image of the obtained tellurium sample, which clearly shows that the obtained crystallites have a wire-like morphology. The diameters of the Te nanowires are not very uniform and the average diameter is calculated to be about 80 nm and lengths are up to tens of micrometers. Figure 3b and its inset display a single Te nanowire and its SAED pattern, which indicates that the nanowire might have [001] directional preferred growth. All of the results clearly show that by using alginic acid as the reducing agent elemental tellurium could be obtained under mild hydrothermal conditions and the formed tellurium nanocrystallites have one-dimensional wire-like morphology. 
Figure 3. TEM images and SAED pattern of the obtained Te nanowires under biomolecule-assisted hydrothermal conditions. Sol-Gel Method For The Growth of SBA-15 Nanorods Array Inside Porous Alumina Membrane Mesoporous materials are special nanomaterials with ordered uniform nanochannels and would have important applications in various fields such as separation, catalysis, adsorption, advanced nanomaterials, etc.[45, 46]. SBA-15 has a highly ordered 2D hexagonal structure with adjustable pore size from 3 to 30 nm and high hydrothermal and thermal stability[46] and is expected to be useful in the synthesis of ultrafine nanorod arrays. However, so far SBA-15 is still in its powder form or as menbrane with channels normally lying in the plane of the substrate, which limits its applications[36]. As an effective template, porous alumina membranes have stimulated great interest for the growth of ordered 1D nanostructures within their pores and up to now many nanorod arrays have been synthesized using porous alumina membrane as growth-limiting template[35]. Compared with SBA-15, alumina membranes have vertical one-dimensional (1D) channel structures, but with the pore sizes in the range of dozens of nanometers to several hundreds of nanometers, which limits its applications in the fabrication of nanodevices. To combine the advantages of the alumina membranes and SBA-15 to form a membrane with fine, vertical mesochannels of about a few nanometers in size is of much importance and would provide wider applications in nanodevice fabrication and more extensive applications in other fields such as separation of biomolecules. Herein we report a simple method for the synthesis of SBA-15 nanorod arrays inside the alumina membrane. 

Figure 4. SEM images of the obtained alumina membrane with SBA-15 nanorods inside. Figure 4 shows the SEM images of the obtained product. The top view SEM image (shown in Figure 4a) of the product evidently shows that nanorods are grown inside the pores of alumina membrane. The diameters of the nanorods are in the range from 200 to 250 nm. Figure 4b is the side view SEM image of the product, displaying that a number of nanorods grew inside the hexagonally arranged pore arrays of alumina. These results clearly confirm that ordered SBA-15 nanorod arrays have formed in the channels of alumina membrane. The mesoporous structure of SBA-15 was shown by TEM images. Figure 5 shows TEM images of the obtained product, clearly displaying that the nanorods inside the porous alumina membrane have parallelly arranged channels with periodic spacing of ~ 9 nm, which is the (100) spacing of SBA-15. The pore diameters of SBA-15 nanorods are calculated to be about 6 nm, which is the typical pore size for SBA-15 mesostructures. From the above results, it is obvious that the SBA-15 nanorod arrays with vertical mesochannels are successfully obtained in the pores of alumina membrane that are used as a template. 

Figure 5. TEM images of the obtained alumina membrane with SBA-15 nanorods inside. Mesoporous SBA-15 As A Hard Template for The Growth Of Pt Nanowires Figure 6 shows Pt nanowires of about 6 nm in diameter and about 100 to 200 nm in length grown in SBA-15. It is very difficult to get smooth and long nanowires using mesoporous materials because of the difficulty in filling the mesopores completely with metal ions. However, better nanowires can be obtained using biomolecule-assisted soft template method as was demonstrated with Te nanowires (See Figure 3). 
Figure 6. TEM image of Pt nanowires using mesoporous SBA-15 as hard template. Conclusions In this review paper, some nanomaterials with controlled size and shape are successfully synthesized under microwave-assisted solvothermal or biomolecule-assisted hydrothermal method. Hexagonally-ordered uniform spherical Ag nanoparticles of about 10 nm were obtained by a one-step interface reaction using dodecylthiol and ethylene glycol under microwave-assisted solvothermal conditions. Pt and Pd nanoparticles were also synthesized under microwave-assisted solvothermal conditions at low temperatures using ethanol or methanol as reducing agents. Under biomolecule-assisted conventional hydrothermal conditions, nanowires of elemental Te were synthesized with alginic acid as reducing agent and morphology-directing agent. SBA-15 nanorod arrays with oriented mesochannels were obtained by using porous alumina membrane as template and this new and efficient mold is expected to find super-function in nanowire fabrication. Platinum nanowires can be grown using SBA-15 as a hard template but the wires appear to be of poor quality. These results show that by the conventional and microwave-assisted hydro or solvothermal methods, nanomaterials with adjustable size and shape can be successfully synthesized. Acknowledgements This work was supported by the NSF MRSEC under grant number, DMR-0213623 and the Huck Institutes of the Life Sciences. TEM work was performed in the electron microscopy facility of the Materials Research Institute at Penn State University. References 1. S. K. Haram, B.M. Quinn and A. J. Bard, “Electrochemistry of CdS nanoparticles: A correlation between optical and electrochemical band gaps”, J. Am. Chem. Soc., 123 (2001) 8860-8861. 2. K. B. Tang, Y. T. Qian, J. H. Zeng and X. G. Yang, “Solvothermal route to semicouductor nanowires”, Adv. Mater., 15 (2003) 448-450. 3. B. D. Busbee, S. O. Obare and C. J. Murphy, “An improved synthesis of high-aspect-ratio gold nanorods”, Adv. Mater., 15 (2003) 414-416. 4. G. Viau, R. Brayner, L. Poul, N. Chakroune, E. Lacaze, F. Fiévet-Vincent and F. Fiévet, “Ruthenium nanoparticles: Size, shape, and self-assemblies”, Chem. Mater., 15 (2003) 486-494. 5. R. He, X. F. Qian, J. Yin and Z. K. Zhu, “Formation of silver dendrites under microwave irradiation”, Chem. Phys. Lett., 369 (2003) 454-458. 6. C. Petit, P. Lixon and M. P. Pileni, “In-situ synthesis of silver nanoculster in AOT reserse micelles”, J. Phys. Chem., 97 (1993) 12974-12983. 7. J. R. Heath, C. M Knobler and D. V. Leff, “Pressure/temperature phase diagrams and superlattices of organically functionalized metal nanocrystal monolayers: The influence of particle size, size distribution, and surface passivant”, J. Phys. Chem. B, 101 (1997) 189-197. 8. L. M. Liz-Marzán and I. Lado-Touriňo, “Reduction and stabilization of silver nanoparticles in ethanol by nonionic surfactants”, Langmuir, 12 (1996) 3585-1589. 9. S. Komarneni, D. Li, B. Newalkar, H. Katsuki and A. S. Bhalla, “Microwave-polyol process for Pt and Ag nanoparticles”, Langmuir, 18 (2002) 5959-5962. 10. I. P. Santos and L. M. Liz-Marzán, “Formation of PVP-Protected metal nanoparticles in DMF”, Langmuir, 18 (2002) 2888-2894. 11. S. A. Harfenist, Z. L. Wang, M. M. Alvarez, I. Vezmar and R. L. Whetten, “Highly oriented molecular Ag nanocrystal arrays”, J. Phys. Chem., 100 (1996) 13904-13910. 12. R. M. Stiger, S. Gorer, B. Craft and P. M. Penner, “Investigations of electrochemical silver nanocrystal growth on hydrogen-terminated silicon (100)”, Langmuir, 15 (1999) 790-798. 13. V. G. Pol, D. N. Srivastava, O. Palchik, V. Palchik, M. A. Slifkin, A. M. Weiss and A. Gedanken, “Sonochemical deposition of silver nanoparticles on silica spheres”, Langmuir, 18 (2002) 3352-3357. 14. T. Itakura, K. Torigoe and K. Esumi, “Preparation and characterization of ultrafine metal particles in ethanol by UV irradiation using a photoinitiator”, Langmuir, 11 (1995) 4129-4134. 15. J. P. Abid, A. W. Wark, P. F. Brevet and H. H. Girault, “Preparation of silver nanoparticles in solution from a silver salt by laser irradiation”, Chem. Commun., (2002) 792-793. 16. K. R. Brown, D. G. Walter and M. J. Natan, “Seeding of colloidal Au nanoparticle solutions. 2. Improved control of particle size and shape”, Chem. Mater., 12 (2000) 306-313. 17. T. Yamamoto, Y. Wada, T. Sakata, H. Mori, M. Goto, S. Hibino and S. Yanagida, “Microwave-assisted preparation of silver nanoparticles”, Chem. Lett., 33 (2004) 158-159. 18. I. Willner, F. Patolsky and J. Wasserman, “Photoelectrochemistry with controlled DNA-cross-linked CdS nanoparticle arrays”, Angew. Chem., Int. Ed. Engl., 40 (2001) 1861-1864. 19. S. Stoeva, K. L. Klabunde, C. M. Sorensen and I. Dragieva, “Gram-scale synthesis of monodisperse gold colloids by the solvated metal atom dispersion method and digestive ripening and their organization into two- and three-dimensional structures”, J. Am. Chem. Soc., 125 (2003) 2305-2311. 20. M. Trau, D. A. Saville and I. A. Aksay, “Field-induced layering of colloidal crystals”, Science, 272 (1996) 706-709. 21. L. O. Brown and J. E. Hutchison, “Controlled growth of gold nanoparticles during ligand exchange”, J. Am. Chem. Soc., 121 (1999) 882-883. 22. C. Petit, A. Taleb and M. P. Pileni, “Cobalt nanosized particles organized in a 2D superlattice: Synthesis, characterization, and magnetic properties”, J. Phys. Chem. B, 103 (1999) 1805-1810. 23. K. Soulantica, A. Maisonnat, M. C. Fromen, M. J. Casanove, P. Lecante and B. Chaudret, “Synthesis and self-assembly of monodisperse indium nanoparticles prepared from the organometallic precursor [In(eta(5)-C5H5)]”, Angew. Chem., Int. Ed. Engl., 40 (2001) 448-451. 24. T. Hyeon, S. S. Lee, J. Park, Y. Chung and H. B. Na, “Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process”, J. Am. Chem. Soc., 123 (2001) 12798-12801. 25. J. S. Yin and Z. L. Wang, “Ordered self-assembling of tetrahedral oxide nanocrystals”, Phys. Rev. Lett., 79 (1997) 2570-2573. 26. S. O’Brien, L. Brus and C. B. Murray, “Synthesis of monodisperse nanoparticles of barium titanate: Toward a generalized strategy of oxide nanoparticle synthesis”, J. Am. Chem. Soc., 123 (2001) 12085-12086. 27. J. T. Hu, L. S. Li, W. D. Yang, L. Manna, L. W. Wang and A. P. Alivisatos, “Linearly polarized emission from colloidal semiconductor quantum rods”, Science, 292 (2001) 2060-2063. 28. L. Motte, F. Billoudet, E. Lacaze, J. Douin and M. P. Pileni, “Self-organization into 2D and 3D superlattices of nanosized particles differing by their size”, J. Phys. Chem. B, 101 (1997) 138-144. 29. F. Gao, Q. Y. Lu and D. Y. Zhao, “Controllable assembly of ordered semiconductor Ag2S nanostructures”, Nano Lett., 3 (2003) 85-88. 30. M. S. Gudiksen, L. J. Lauhon, J. Wang, D. C. Smith and C. M. Lieber, “Growth of nanowire superlattice structures for nanoscale photonics and electronics”, Nature, 415 (2002) 617-620. 31. C. N. R. Rao, A. Govindaraj, F. L. Deepak, N. A. Gunari and M. Nath, “Surfactant-assisted synthesis of semiconductor nanotubes and nanowires”, Appl. Phys. Lett., 78 (2001) 1853-1855. 32. Z. H. Wu, M. Sun, X. Y. Mei and H. E. Ruda, “Growth and photoluminescence characteristics of AlGaAs nanowires”, Appl. Phys. Lett., 85 (2004) 657-659. 33. J. Choi, G. Sauer, K. Nielsch, R. B. Wehrspohn and U. Gosele, “Hexagonally arranged monodisperse silver nanowires with adjustable diameter and high aspect ratio”, Chem. Mater., 15 (2003) 776-779. 34. Y. J. Han, J. M. Kim and G. D. Stucky, “Preparation of noble metal nanowires using hexagonal mesoporous silica SBA-15”, Chem. Mater., 12 (2000) 2068-2069 35. C. M. Zelenski and P. K. Dorhout, “Template synthesis of near-monodisperse microscale nanofibers and nanotubules of MoS2”, J. Am. Chem. Soc., 120 (1998) 734-742. 36. Y. F. Lu, R. Ganguli, C. A. Drewien, M. T. Anderson, C. J. Brinker, W. L. Gong, Y. X. Guo, H. Soyez, B. Dunn, M. H. Huang and J. I. Zink, “Continuous formation of supported cubic and hexagonal mesoporous films by sol gel dip-coating”, Nature, 389 (1997) 364-368. 37. Q. Y. Lu, F. Gao and S. Komarneni, “Biomolecule-Assisted Reduction in the Synthesis of Single-Crystalline Tellurium Nanowires”, Adv. Mater., 16 (2004) 1629-1632. 38. Q. Y. Lu, F. Gao and S. Komarneni, “Ordered SBA-15 nanorod arrays inside a porous alumina membrane”, J. Am. Chem. Soc., 126 (2004) 8650-8651. 39. C. M. Niemeyer, “Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science”, Angew. Chem., Int. Ed. Engl., 40 (2001) 4128-4158. 40. C. Mao, D. J. Solis, B. D. Reiss, S. T. Kottmann, R. Y. Sweeney, A. Hayhurst, G. Georgiou, B. Iverson and A. M. Belcher, “Virus-based toolkit for the directed synthesis of magnetic and semiconducting nanowires”, Science, 303 (2004) 213-217. 41. M. Knez, A. M. Bittner, F. Boes, C. Wege, H. Jeske, E. Maiβ and K. Kern, “Biotemplate synthesis of 3-nm nickel and cobalt nanowires”, Nano Lett., 3 (2003) 1079-1082. 42. A. I. Usov, “Alginic acids and alginates: analytical methods used for their estimation and characterisation of composition and primary structure”, Russ. Chem. Rev., 68 (1999) 957-966. 43. B. Mayers and Y. Xia, “Formation of tellurium nanotubes through concentration depletion at the surfaces of seeds”, Adv. Mater., 14 (2002) 279-282. 44. Z. Liu, Z. Hu, Q. Xie, B. Yang, J. Wu and Y. Qian, “Surfactant-assisted growth of uniform nanorods of crystalline tellurium”, J. Mater. Chem., 13 (2003) 159-162. 45. C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, “Ordered mesoporous molecular-sieves synthesized by a liquid-crystal template mechanism”, Nature, 359 (1992) 710-712. 46. D. Y. Zhao, J. P. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, “Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores”, Science, 279 (1998) 548-552. Contact Details |