Multiple techniques may be needed to characterize key parameters of different lots of heparin. Weight-average molar mass measured by multi-angle static light scattering (MALS) and hydrodynamic radius measured by dynamic light scattering (DLS) can be used to characterize the size and polydispersity of the molecule.
It is possible to use these quantities as metrics for lot-to-lot comparison and to qualify heparin from different suppliers. Massively-parallel phase analysis light scattering (MP-PALS) and DLS are used for calculating the net charge and for characterizing the purity of the sample. This study compares heparin from two suppliers including one lot modified with super-sulfated material.
Results and Discussion
Size exclusion chromatography coupled with MALS determined weight-average molar mass (Mw) for the different heparin samples and for heparin mass standards. The difference in Mw and hydrodynamic radius (rh) between the two suppliers was <10%. Very little difference in Mw and rh (<5%) was observed between unmodified heparin and heparin from the same supplier modified with super-sulfated material. Hence size alone could not be used for qualifying contaminated heparin samples.
Using the Wyatt Möbius, z-averaged electrophoretic mobility and rh were measured simultaneously to obtain the net charge of the heparin molecules. The charge determined for these unfractionated samples was compared to the measured charge for a panel of heparin molar mass standards. It was seen that there was a linear relationship between effective molecular charge and molecular weight, indicating a constant charge:mass ratio.
The same linear relationship was obeyed by unmodified heparin from both suppliers, but the super-sulfated material exhibited a 30% increase in negative charge. The increase in negative charge per unit mass, is consistent with an increase in sulfate groups in the modified heparin sample.
Hence the combination of Mw by SEC-MALS and net charge by MP-PALS provides clear differentiation between unmodified and contaminated heparin. This multi-technique approach for determining the charge:mass ratio enables rapid, non-destructive characterization of different lots of heparin. Figure 1 shows the molecular structure for heparin.
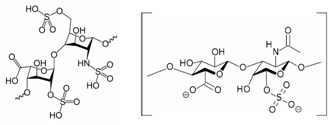
Figure 1. Molecular structure for heparin (left) and chondroitin sulfate (right). The sulfate groups are expected to impart an increased negative charge on heparin samples contaminated with chondroitin sulfate.
Baxter International, Inc. provided the unfractionated heparin samples and fractionated heparin standards (Heparin Derived Polysaccharides, Neoparin, Inc.). Samples were dissolved in 0.1M ammonium acetate to a final concentration of ~5mg/mL and allowed to equilibrate overnight at room temperature prior to beginning analyses.
MP-PALS analysis was performed using the Wyatt Möbius; samples were filtered to 0.02µm using a syringe tip filter (Anotop, Whatman) as they were injected directly into the Möbius; flow cell. Electrophoretic mobility by massively-parallel phase analysis light scattering (MP-PALS) was measured simultaneously with hydrodynamic radius by dynamic light scattering (DLS).
Materials and Methods
Weight-average molar mass (Mw) for each sample was determined using multi-angle static light scattering (MALS). Molar mass and polydispersity of fractionated heparin samples was measured by size exclusion chromatography (SEC) coupled with MALS. Unfiltered samples were injected onto a chromatography column, and the molar mass of the eluting sample was measured using a DAWN HELEOSMALS detector and Optilab rEX refractive index detector. SEC-MALS data for unfractionated heparin was provided by Baxter International, Inc.
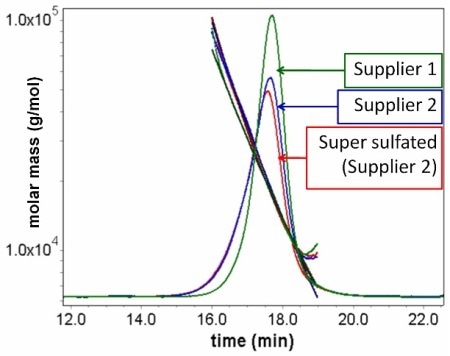
Figure 2. Overlay of SEC-MALS data for heparin from two different suppliers, including a one sample that has been contaminated with “super-sulfated” material.
As shown in Table 1, the difference in molar mass (5%) between the pure heparin and heparin contaminated with super-sulfated material from Supplier 2 was less than the difference in molar mass of the pure heparin coming from two different suppliers (18%). Furthermore the hydrodynamic radius of the pure heparin is indistinguishable from the sample contaminated with super-sulfated material.
Table 1. Weight-average molar mass and z-average hydrodynamic radius of unfractionated heparin samples.
| |
Mw (kDa) |
rh (nm) |
| Unfractionated Heparin, Supplier 1 |
15.9 ± 0.1 |
2.30 ± 0.02 |
| Unfractionated Heparin, Supplier 2 |
18.8 ± 0.3 |
2.48 ± 0.01 |
| Super-sulfated Heparin, Supplier 2 |
17.9 ± 0.0 |
2.49 ± 0.02 |
Figure 3 shows a typical “V-graph” for the measurement of the electrophoretic mobility. The negative electrophoretic mobility (µ) indicates the heparin sample has a negative net charge. The effective molecular charge and zeta potential are then calculated from the electrophoretic mobility and hydrodynamic charge and weight-average radius. These parameters are summarized for the unfractionated heparin samples and fractionated samples (NHP) in Table 2.
Table 2. Electrophoretic mobility, net charge, and zeta potential measured by MP-PALS.
| |
Mobility ((µm*cm)/(s*V)) |
Effective charge (Z*) |
Zeta Potential (mV) |
rH (nm) |
| Unfractionated Heparin, Supplier 1 |
-1.00 ± 0.05 |
-7.1 ± 0.40 |
-16.9 ± 0.9 |
2.30 ± 0.02 |
| Unfractionated Heparin, Supplier 2 |
-1.18 ± 0.09 |
-9.3 ± 0.64 |
-19.5 ± 1.5 |
2.48 ± 0.01 |
| Super-sulfated Heparin, Supplier 2 |
-1.46 ± 0.12 |
-11.2 ± 0.96 |
-23.8 ± 1.9 |
2.49 ± 0.02 |
| NHP III |
-0.42 ± 0.03 |
-3.2 ± 0.26 |
-7.9 ± 0.57 |
2.19 ± 0.02 |
| NHP IV |
-0.65 ± 0.10 |
-4.2 ± 0.56 |
-11.8 ± 1.7 |
2.09 ± 0.02 |
| NHP VI |
-0.86 ± 0.05 |
-7.1 ± 0.39 |
-15.8 ± 0.9 |
2.43 ± 0.02 |
| NHP VII |
-0.85 ± 0.10 |
-14.2 ± 1.8 |
-14.2 ± 1.7 |
3.74 ± 0.03 |
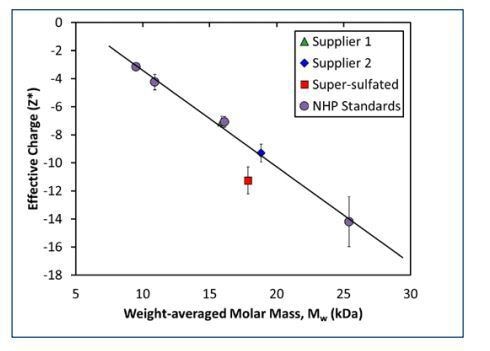
Figure 3. Measured net charge and molar mass for heparin standards exhibits a linear relationship, indicating a constant charge:mass ratio. Pure heparin samples from both suppliers obey the same linear relationship, but heparin contaminated with super-sulfated.
Among the three unfractionated samples, the super-sulfated material exhibited the largest net charge. However, the difference in charge between the super-sulfated and pure samples was of the same magnitude as the difference in charge for the two suppliers. The difference might only reflect the variance in molar mass or polydispersity.
The net molar mass of the fractionated heparin standards (NHP III, IV, VI, and VII) were used to generate a calibration curve for pure heparin. These four samples establish a linear relationship between net charge and molar mass for pure heparin, indicating a constant charge:mass ratio. Based on their measured Mw, the net charges for unfractionated heparin from Suppliers 1 and 2 fall within 2% of expected value. However, the measured net charge of the super-sulfated heparin from Supplier 2 is ~30% greater than what would be expected for a heparin of that size.
Conclusion
Simultaneous measurements of electrophoretic mobility and hydrodynamic radius enable rapid, non-destructive characterization of effective molecular charge. The increase in negative charge, as measured by a change in electrophoretic mobility, is consistent with an increase in sulfate groups in the modified heparin sample.
The increase in charge combined with the molar mass of the sample provides a unique fingerprint for pure heparin compared to heparin samples contaminated with super-sulfated material. In this study, the effective charge:mass ratio clearly distinguished between unmodified and super-sulfated heparin and can be used as a metric to qualify different samples.
Reference
Reprinted with Permission from "Discriminating Heparin from Chondroitin Sulfate by Charge: Mass Ratio." White Paper by Wyatt Technology.

This information has been sourced, reviewed and adapted from materials provided by Wyatt Technology.
For more information on this source, please visit Wyatt Technology.