Pharmaceutical companies follow quality control (QC) measures and depend on microscope-based studies to detect, measure, and qualify solid particles that fulfill the required size parameters. For instance, the specification may restrict the number of solid particles to 100% less than 100 µm in length and 90% less than 25 µm in length. This approach can also define the amount of product to be utilized in the test.
In current practice, the analyst scans the sample and the total number of particles is estimated together with the percentage of solid particles satisfying the desired specification. For further sale or processing, the samples are examined prior to releasing the batch of product. However, such manual methods are labor-intensive, take significant amount of time, and are subject to variations from analyst to analyst.
In contrast, the Morphologi particle characterization system can easily automate such procedures and expedite the analysis time. This not only reduces the errors caused by human subjectivity, but also reduces operators’ time. In addition, the system can characterize particles as per their classifications and shape and can be easily set up to detect the number of needle- and circular-shaped particles in a sample.
Counting Needle-Shaped Crystals in Pharmaceutical Ointment
In one particular example, the Morphologi system is utilized to automate the manual microscopy quality control measures, which involve assessing the number of needle-like particles of an API (active pharmaceutical ingredient) in a commercially available pharmaceutical ointment. In this ointment, one of the actives form thin and long needle-shaped crystals in a stable state with actives suspended in the ointment, as shown in Figure 1.

Figure 1. Image of needle-like crystals in the pharmaceutical ointment.
In this product, quality control specifications based on microscopy approach specifies that in a sample of 3mg, particles beyond 250 µm in length should not be present. Also, only 100 particles longer than 100 µm should be present. The amount of particles ranging from 50 to 100 µm in length is also taken into consideration for information purposes.
In contrast to present manual microscopy analysis that consumes considerable amount of time, automating the measurement on the Morphologi system reduced the time taken for sample analysis to below 15 minutes. The needle-like crystals were detected, quantified and classified without human subjectivity variations. Another benefit of Morphologi is that for all the analyzed particles, a permanent record can be maintained.
Automating the Measurement with Particle Characterization System
The paraffin media which serves as the base of the ointment makes it difficult to detect needle-like particles by microscopic technique at room temperature. When the sample is heated, the paraffin base is melted and makes it easy to identify the crystals (Figure 1).
The Morphologi particle characterization system is designed around an advanced microscope platform that determines and characterizes particles with respect to shape and size. The system is highly sensitive and includes a classification feature that enables particles, which satisfy the specified standards, to be counted as part of that class.
For sample analysis, a standard operating procedure (SOP) was set up. The SOP includes all the hardware and software variables required for the measurement. This way, each measurement is carried out in the same manner. In addition, since the SOP can be transferred between systems, it prevents variations that result from site to site, or user to user.
The sample was heated to 65°C between a coverslip and a microscope slide on a hot plate. Then, the slide was placed on the slide holder of the Morphologi system and a heat gun controlled by temperature was utilized to keep the sample temperature at 65°C. The samples were measured in line with the SOP by scanning the specified area and simultaneously examining the particles in real time. Furthermore, the Morphologi logs the x,y-coordinates of each particle and thus make sure that none of the particles are counted again. The time taken for this measurement was less than 10 minutes. Different lengths of the needle-shape particles were grouped together using the classification function. Table 1 shows the defined classes.
Table 1. Classification criteria set up for automated analysis.
| Class |
Specification |
| = 250 µm |
Length = 250 µm Aspect ratio < 0.33 |
| = 100 < 250 µm |
Length = 100 < 250 µm Aspect ratio < 0.33 |
| = 50 < 100 µm |
Length = 50 < 100 µm Aspect ratio < 0.33 |
Images of all the particles are stored after the measurement is over, and individual particles from each class can be observed in the particle view. In case users want to study the individual particles at a higher magnification, they can simply click on the particle present in the particle view and return to it in the sample from the x,y-coordinates of each particle. In the records view, the results of all the analyzes are summarized and can be shown as a table in a customizable report (Table 2). Reports show the particles in particular classes can also be customized (Figure 2).
Table 2. Summary of particles counted in each class defined in the SOP.
| Sample name |
>250 µm |
100-250 µm |
50-100 µm |
| Sample 1 |
0 |
4 |
63 |
| Sample 2 |
0 |
3 |
126 |
| Sample 3 |
0 |
69 |
286 |
| Sample 4 |
0 |
4 |
26 |
| Sample 5 |
0 |
26 |
136 |
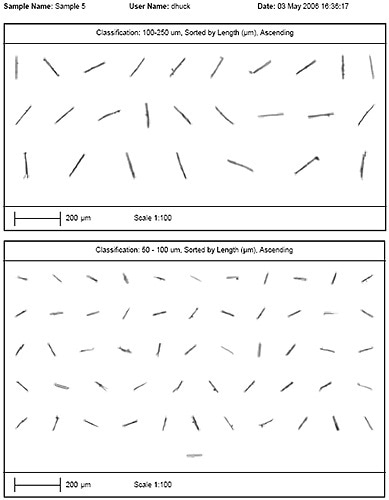
Figure 2. Example of a particle report.
Conclusion
The Morphologi is an automated image analysis instrument that helps in automating quality control microscope procedures, which earlier has been carried out manually. The system can be used to detect and count particles that fulfill certain shape and size parameters. This approach reduces measurement time, frees up operators’ time, and considerably reduces human subjectivity variations.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information on this source, please visit Malvern Panalytical.