Biological samples come in all shapes and sizes, for example, tough and fibrous plants, hard bones, soft muscles, tough and viscous sputum, or tumor or liver tissue. In addition, there are millions of cell-like bacteria, yeast, or algae, which have to be disrupted for applications such as protein extraction or RNA or DNA isolation.
As sample preparation is the primary step of every analytical process, all types of biological samples have to be prepared for research in metabolomics, genomics, or transcriptomics. RETSCH provides a series of grinders and mills for easy and reproducible pulverization of solid sample materials, of which some are also ideal for cell disruption and homogenization of biological sample materials.
In this article, the use of RETSCH mills is described for the following applications:
- Cell disruption of microalgae, yeasts and bacteria (in suspension)
- Cryogenic grinding of pine needles, cell pellets, and muscle tissue
- Homogenization of tough and soft biological samples such as liver or sputum
- Washing procedure to acquire intact bacterial cells from infected human tissue
- Pulverization of forensic samples — hair, teeth and bones
Cell Disruption of Microalgae, Bacteria and Yeasts (in Suspension)
A standard procedure in basic biological research, medical research, or applied biotechnology is cell disruption of yeast, bacteria, microalgae, or filamentous fungi to get access to cell proteins, metabolites, or nucleic acids (DNA, RNA). Different techniques of cell disruption can be used on a lab scale which can be generally classified into techniques using chemicals to destroy the cell membranes and structures, and mechanical methods.
Using mechanical methods, the thick walls of cells such as Bacillus spores and Mycobacterium cells, which are difficult to lyse, can be broken. There is no need for the addition of chemicals which might affect the subsequent extraction steps.
Bead beating is an extensively used, effective, and straightforward mechanical method for cell disruption, which involves beads made of ceramics, steel, or glass. The beads are thoroughly mixed with the cell suspension by shaking or stirring; due to the large surface area of the glass beads shearing forces break the cell walls, resulting in a release of cellular components. Although bead beating is usually used for small-scale cell disruption in 2 ml single-use vials, it can also be employed for larger vials, like 50 ml disposable Falcon tubes.
The benefits of this method over other mechanical methods, like ultrasonification, include the possibility to process various samples in a single step without the risk of cross-contamination, ease of use, and the better yield of cellular components. The most straightforward technique for bead beating is to mix the cell suspension with an equal volume of beads with the help of a laboratory vortex mixer for stirring.
However, this technique is error-prone and time-intensive, particularly for cell disruption times up to 10 minutes, or for high sample throughput. The process can be made fast, reproducible, and efficient using RETSCH’s Mixer Mill MM 400 in combination with various adapters. Since the cells are automatically broken, the operator can save time that can be used for further steps in the analytical process.
In general, less than 1 ml of cell material is required for the isolation of DNA or RNA, hence cell disruption is primarily performed using 1.5 ml or 2 ml Eppendorf® tubes. RETSCH provides distinct adapters for these vials, thereby enabling up to 20 samples to be processed in a single step. However, for the extraction of metabolites or proteins, greater amounts of cell suspension are needed, therefore it is advisable to perform cell disruption in 50 ml disposable tubes (Falcon® tubes); the MM 400 can be used to simultaneously process eight samples (eight samples of 30 ml cell suspension or, if needed, 240 ml cell suspension divided to eight conical centrifuge tubes with subsequent cell suspension pooling). Currently, the missing size between 2 ml and 50 ml tubes is available with the new 5 ml Eppendorf® tubes, which can also be used with the MM 400.
Case Study: Cell Disruption of Saccharomyces Cerevisiae
For studying, e.g. the functionality of cell proteins, it is essential to disrupt volumes of roughly 30 ml cell suspension. The automated process using the Mixer Mill MM 400 together with the adapter for conical centrifuge tubes (eight samples simultaneously) was compared to a vortexer with manual cell disruption. In order to obtain a sufficient protein yield, vortexing 4 g of yeast cells with 16 g glass beads (0.5–0.75 mm) and 12 g disruption buffer in 50 ml tubes takes 12 x 1 min with 1 min intermediate cooling on ice.
Disruption of more than two samples at the same time can only be performed by making several people work on a number of vortexers in a row. Furthermore, it is obligatory to exchange the samples among the operators to make up for hardware-specific as well as user-specific differences.
Lab work is greatly simplified by the use of the Mixer Mill MM 400. This mill can be used to automatically disrupt various samples of cell suspension with a frequency up to 30 Hz. It is possible to reduce the time needed for cell disruption to 7 minutes to realize an even better protein yield compared to vortexing after 12 minutes (Figure 1). Another benefit of the automated process using the MM 400 is the minimal increase in temperature in the cell suspension at the time of cell disruption and the improved reproducibility (Figures 2 and 3).
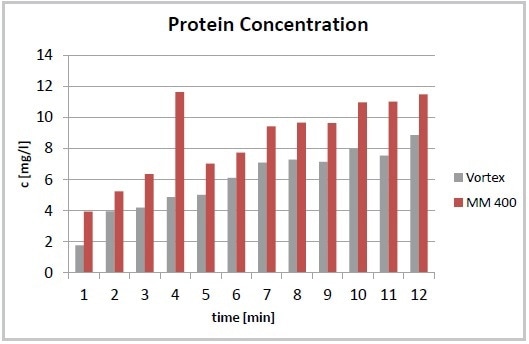
Figure 1. Total protein concentration after cell disruption using a Vortexer and the Mixer Mill MM 400. The required yield of proteins is achieved after 7 minutes (MM 400) compared to 12 minutes required by vortexing.
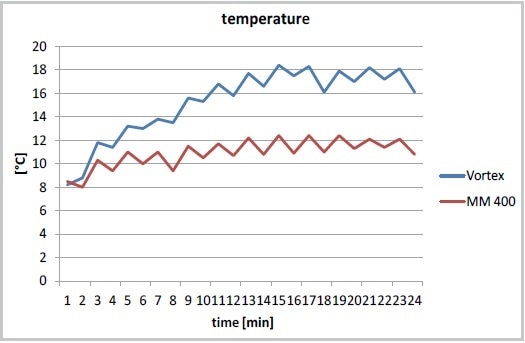
Figure 2. Temperature increase during cell disruption in the MM 400 (at 30 Hz) or with a Vortexer, cooling on ice after each minute of cell disruption.
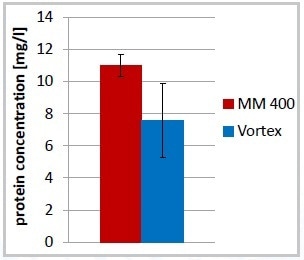
Figure 3. Increased reproducibility of total protein concentration after cell disruption, 7 minutes in MM 400, 12 minutes Vortex; error bars: % standard deviation.
Optimization of the yeast cell disruption was achieved by modifying the bead size and the oscillating frequency. While extracting proteins, it was advantageous to decrease the speed to 20 Hz, leading to a slight increase in total protein content and less foaming. Furthermore, the increase in temperature was less at 20 Hz compared to 30 Hz (Figure 4).
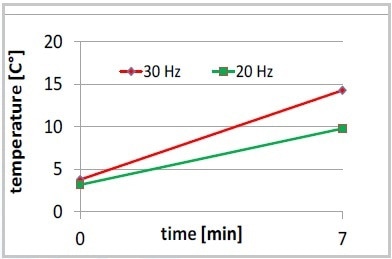
Figure 4. Increase of temperature, 7 minutes MM 400 at 20 or 30 Hz, no intermediate cooling on ice.
There was a marginal improvement in the results of cell disruption when glass beads sized 0.75–1.00 mm were used instead of those sized 0.5–0.75 mm and by increasing the mass to 24 g (Figure 5).
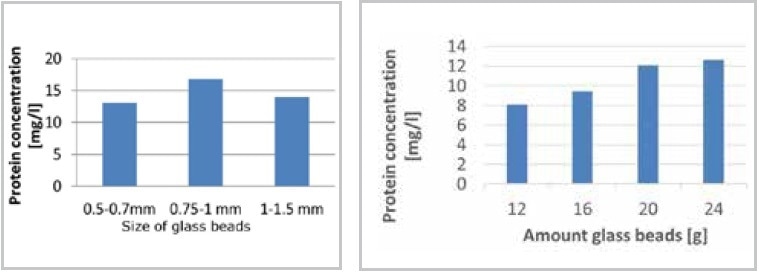
Figure 5. Optimization of cell disruption in the MM 400. Using a bead size range from 0.75 to 1 mm results in highest protein concentration (left); the protein concentration is influenced by the number of beads (right).
In another method, the process of yeast cell disruption performed using the MM 400 was compared with ultrasonification. Moreover, a comparison was made between the protein concentrations following cell disruption in 50 ml conical centrifuge tubes and 2 ml single-use tubes. After 5 minutes, the protein yield realized using bead beating was nearly six times higher than the yield realized using ultrasonification (results not shown). The difference between 25 ml cell suspension (centrifuge tubes) or 1 ml cell suspension (2 ml tubes) was insignificant.
The evident benefits of the automatic cell disruption process over other techniques such as ultrasonification or manual vortexing have been clearly demonstrated. The technique allows simultaneous processing of several samples, offers a high protein yield, maintains the increase in temperature of the cell suspension during disruption within tolerable limits, and enables excellent reproducibility.
Case Study: Cell Disruption of Microalgae
Cell disruption of microalgae, such as diatoms, could be difficult since the cells tend to oppose the shearing effects generated by the glass beads. In the past it has not been possible to successfully perform cell disruption of diatoms using glass beads together with shakers, a French Press was typically used instead. When compared to other shakers, the Mixer Mill MM 400, together with the adapter for conical centrifuge tubes is best suited to effectively disrupt microalgae cells.
About 300 ml of cell suspension of the organism Thalassiosira pseudonana was centrifuged, re-suspended in 20 ml disruption buffer, and filled into a 50 ml conical centrifuge tube; 40 ml glass beads (sized 90–150 μm and 300–400 μm, ratio 1:1) were added and cell disruption was carried out for 20 seconds at 20 Hz. The complete cell disruption could be observed using a microscope (Figure 6).
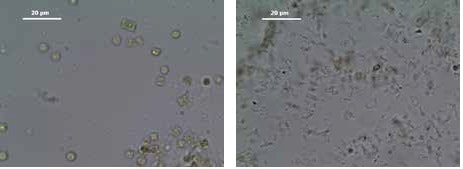
Figure 6. Cells of the diatom Thalassiosira pseudonana before (left) and after disruption (right) using the Mixer Mill MM 400 in combination with the tube adapter for 50 mL tubes; 20 seconds at 20 Hz.
The MM 400 was able to realize even complete cell disruption of the algae Phaeodactylum tricornutum, which easily opposes shearing effects due to a missing silicate cell wall. A cell culture of 200 ml was centrifuged, re-suspended in 20 ml disruption buffer, and transferred to a 50 ml conical tube. Glass beads of 40 ml (sized 90–150 μm and 300–400 μm, ratio 1:1) were added and cell disruption was carried out within 3 x 60 seconds at 30 Hz. After 3 min in total, no intact cells could be observed using a microscope (Figure 7).
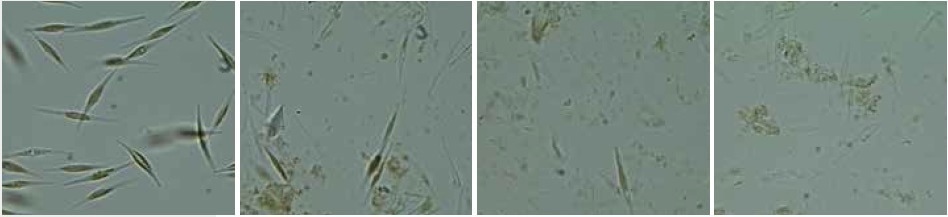
Figure 7. Cells of Phaeodactylum tricornutum before (left) and after cell disruption (middle and right) with the Mixer Mill MM 400 in combination with the adapter for conical centrifuge tubes; 3 x 60 seconds at 30 Hz.
In contrast to the technique that involves using a French Press, the bead beating process using the MM 400 is less time-consuming, as it is possible to simultaneously disrupt eight samples, and the risk of cross-contamination is minimized. Using the MM 400 instead of other shakers is recommendable as it produces shearing forces strong enough to effectively disrupt even those cells that usually oppose disruption through bead beating.
Homogenization of Tough and Soft Biological Samples such as Liver or Sputum
Occasionally, the preparation and homogenization of biological samples can be as tough as the material itself. The 2 ml single-use tubes typically used are generally not large enough to incorporate the whole sample volume; therefore, it is essential for the sample to be divided and reunited following the homogenization process, which means performing an extra time-consuming working step in the lab routine. Despite the availability of larger sized grinding jars — for example, made of stainless steel — that can incorporate the entire sample volume, their drawback is that they have to be cleaned after use.
The ideal solution to this problem are the new 5 ml single-use tubes which do not need to be cleaned. RETSCH has developed a new adapter for the Mixer Mill MM 400, which accommodates five of the 5 ml tubes, thereby allowing the preparation of 10 x 5 mL of sample at the same time.
The nature of biological samples can vary greatly, for instance, extremely tough sputum of cystic fibrosis patients or tissue samples such as lung, liver, or tumors. Below, the use of the MM 400 in combination with the new adapter for 5 ml single-use tubes for homogenization of these samples is described.

Figure 8. Adapter for MM 400 which accommodates five 5-mL tubes.
Case Study: Homogenization of Very Tough Sputum of Cystic Fibrosis Patients
The sputum that cystic fibrosis patients cough up is frequently homogenized for routine diagnostics as well as for research. The Mixer Mill MM 400 can be used to easily homogenize the sputum sample; however, since 2 ml vials were used in the past, it was not feasible to process more than 1 ml of each sputum sample. It was not an appropriate procedure to divide the viscous and tough sputum into portions for homogenization as it is a partly infectious material. An alternative option was the use of 5 ml stainless steel grinding jars, which necessitated meticulous cleaning. The new adapter for 5 ml reaction tubes offers the combined advantages of higher volume and disposable vials.
During a trial, up to 3 ml was homogenized using two to three zirconium oxide grinding balls (Ø 5 mm) at 30 Hz for almost 2.5 minutes. The possibility to process the entire sample in the 5 ml tubes in a single step, reduces workload and saves time; it also reduces the handling of potentially infectious material to a minimum.
Case Study: Homogenization of Liver Tissue
Tissue samples such as liver, lung, or tumors are routinely investigated in biological basic research, biomedicine, molecular diagnostics, toxicology, pharmacology, and many more areas. The challenges faced by the user here are similar to those of homogenizing sputum. Therefore, the new 5 ml single-use tubes together with the Mixer Mill MM 400 are also a suitable solution for effective homogenization even for this application. Certain combinations of grinding ball numbers and sizes and sample volumes were investigated for homogenizing liver, with the result that a few 10 mm balls had a considerably better size reduction effect on the tissue structure than a number of smaller balls.
The use of three 10 mm stainless steel grinding balls provided optimum results for the thorough homogenization of 3 g of fresh liver tissue. Aqueous buffer was filled into the reaction vial up to the maximum filling line. Then, the sample was homogenized for 5 minutes at 30 Hz and tissue residues could not be observed after the process (Figure 9). It is possible to realize the same degree of homogenization in conical centrifuge tubes with the following parameters: 15 g sample, four stainless steel balls (20 mm), 30 Hz, buffer, 3 minutes. The weight of grinding ball/sample/buffer restricts the number of samples that can be processed at a time using centrifuge tubes to only two. The increase in temperature in both cases is insignificant.

Figure 9. Liver sample before and after homogenization in the MM 400.
Cryogenic Grinding of Cell Pellets, Muscle Tissue, and Pine Needles
Tough, soft, and fibrous biological samples can be pulverized without a buffer. For materials such as very tough veins or fingernails, fibrous plants, or different kinds of animal or tumor tissue, homogenization in a buffer system is not very efficient. However, cryogenic grinding — that is, the embrittling of the samples using dry ice or liquid nitrogen prior to or during milling — has proven to be an ideal method to acquire homogeneously pulverized samples.
In contrast to the bead beating process, the technique of freezing the samples before crushing is also appropriate for cracking intracellular organelles, for instance, of yeast. One more benefit of cryogenic grinding is the extremely low temperature, which prevents the degradation of, for example, proteins or dramatic reduction in the loss of volatile ingredients that may take place at the time of milling. For sticky samples such as berries (Figure 11), cryogenic grinding is usually the only technique to produce a homogeneous sample. Table 1 outlines sample materials that have been successfully pulverized through cryogenic grinding using the Mixer Mill MM 400 or the CryoMill.
Table 1. Different biological samples pulverized by cryogenic grinding with liquid nitrogen
| Sample |
Accessories |
Feed quantity |
Grinding time |
Speed |
Final fineness (d90)/result |
| E. coli bacteria |
- 2 grinding jars stainless steel 50 ml
- 2 grinding balls 25 mm stainless steel per jar
|
2 x 10 ml frozen cell pellets |
2 min |
30 Hz |
complete cell disruption |
| Muscle tissue |
- grinding jar stainless steel 50 ml
- grinding ball 25 mm stainless steel
|
10 g |
4 min |
25 Hz |
<150 μm |
| Pine needles |
- 2 adapters for ten 2 ml reaction vials each
- 2 x balls stainless steel 5 mm per vial
|
2 needles per vial |
3 min |
30 Hz |
Reproducible RNA extraction of 20 samples in one step |
| Berries |
- grinding jar stainless steel 50 ml
- 4 x grinding balls stainless steel 15 mm
|
2 g |
40 secs |
20 Hz |
<200 μm |
| Finger nails |
- adapter CryoMill for 4 x 2 ml vials
- 4 grinding balls stainless steel 5 mm per vial
|
1 finger nail per vial |
2 min |
25 Hz |
<200 μm |
| Rat gut |
- grinding jar stainless steel 35 ml
- 1 x grinding ball stainless steel 20 mm
|
1.8 g |
2 min |
30 Hz |
<150 μm |

Figure 10. Pieces of meat before and after cryogenic grinding.
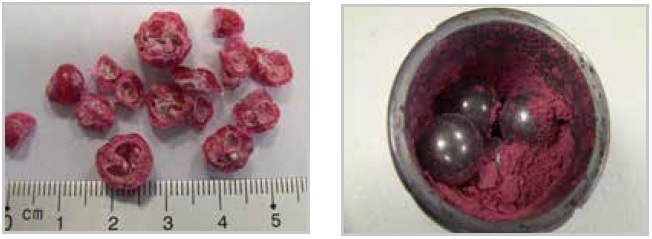
Figure 11. Sticky berries before and after cryogenic grinding.

Figure 12. Pine needles before and after cryogenic grinding (including agarose gel for RNA separation showing good reproducibility and quantity of prepared RNA after grinding).
Suitable Lab Mills for Cryogenic Grinding
The Mixer Mill MM 400 or the CryoMill (Figure 13) can be effectively used for pulverizing small sample quantities. Appropriate grinding jars of the MM 400 are made of PTFE or steel; it is also possible to use single-use vials of 1.5 or 2 ml. Care must be taken to ensure that liquid nitrogen is not enclosed within the grinding jars or tubes since the heat produced by the grinding process turns the liquid nitrogen (LN2) gaseous, leading to an excessive pressure increase inside the grinding jar.
Using tongs, the closed grinding jar is placed inside an insulation container filled with liquid nitrogen for 2–3 minutes and is subsequently clamped into the MM 400. As a result of the high energy input and the ensuing frictional heat, it is necessary to ensure that the grinding process does not take more than 3 minutes to prevent warming up of the sample and to retain its breaking properties. In case longer grinding times are necessary, intermediate cooling of the closed grinding jars should be performed to interrupt these.

Figure 13. CryoMill with 50 L Dewar.
RETSCH has created the CryoMill as an advancement of the MM 400, offering the benefit of constant cooling of the grinding jar with liquid nitrogen, keeping the temperature of the sample and the jar at −196 °C even during long grinding processes. In case intermediate cooling is needed, it is possible to program cycles of grinding and cooling.
Since the grinding jars stay in the mill throughout the entire process and the user does not come into contact with liquid nitrogen at any point of time, the operation of the CryoMill is extremely safe. The automatic pre-cooling function guarantees that the grinding process does not begin before attaining and maintaining a temperature of −196 °C. A zirconium oxide grinding jar can be used with the CryoMill for heavy-metal-free grinding.
Pulverization of Forensic Samples: Hair, Teeth and Bones
Forensic materials like hair, teeth, and bones are often brittle and therefore normally do not need cooling before pulverization. Based on the required analytical fineness, it may be necessary for the samples to be subjected to preliminary crushing in a cutting mill or jaw crusher to generate particle sizes that are sufficiently small for further processing in a ball mill (<10 mm). For pre-cutting bones that may be fresh and therefore not fully dry (and may possibly even contain meat residues), cutting mills are used.
RETSCH provides a complete range of cutting mills (Figure 14) for primary size reduction of tough, soft, elastic, fibrous, and medium-hard sample materials. The broad array of accessories permits perfect adaptation to a range of applications. It is possible to equip the SM 300 with three different rotors and bottom sieves with aperture sizes ranging from 0.25 to 20 mm. When compared to fresh and fatty bones, it may even be possible to reduce the size of the dry bones to <0.25 mm in one or two steps. In order to enable optimum adaptation to sample properties in relation to temperature sensitivity and breaking behavior, the SM 300 offers a variable speed of 100–3000 min-1 and is robust enough to grind even tough bones.

Figure 14. RETSCH offers different types of cutting mills.
Application Example Bones
In this example, 700 g of bones were pre-crushed using the SM 300, in combination with a six-disk rotor and a bottom sieve with an aperture size of 6 mm. The bones were crushed at 3000 min-1 within just 30 seconds, resulting in pieces smaller than 6 mm (Figure 15).
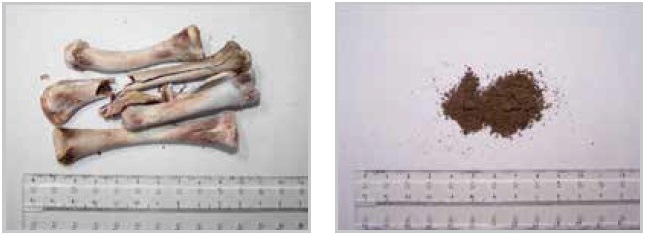
Figure 15. Bone sample before and after grinding in a SM 300 cutting mill.
Pulverization of teeth or bones is often performed in ball mills using grinding balls of sizes >5 mm made of zirconium oxide, steel, or tungsten carbide. The large grinding balls have a crushing power adequate to pulverize these very hard samples through impact. In the example above, the pre-crushed bones were pulverized using the Mixer Mill MM 400; 1 g of the sample was filled into a 35 ml zirconium oxide grinding jar and finely ground with the help of a 20 mm grinding ball made of zirconium oxide.
After performing the process for 2 minutes at 30 Hz, the sample was pulverized to particles with a size less than 200 µm, and hence ready for extraction of nucleic acids. In case the sample is extremely tough, the breaking behavior can be improved by embrittlement with liquid nitrogen.
Application Example Teeth
One more example is the size reduction of teeth. One piece of tooth was added to a 25 ml zirconium oxide grinding jar and pulverized using a grinding ball sized 20 mm and made of the same material. After processing for 3 minutes at 30 Hz in the MM 400, the sample had turned into a powder with the majority of particles measuring smaller than 100 μm (Figure 16, right).

Figure 16. Teeth before and after grinding in the MM 400 mixer mil.
Application Example Hair
The CryoMill, which has been specifically designed for cryogenic grinding, and the MM 400 may also be used for grinding fibrous samples such as hair. Surprisingly, very healthy hair has to be ground under cryogenic conditions; in contrast, unhealthy hair can be milled at room temperature since it breaks more easily. When compared to the very compact teeth or bones, it is more suitable to grind hair using smaller grinding balls, since friction and not impact is needed for the process. It is necessary to pay attention to the grinding time — it must be kept as short as possible since the samples tend to get burnt. For instance, 1 g of hair is added to a 25 ml stainless steel grinding jar including six stainless steel balls with a diameter of 10 mm. After being milled for 2 minutes at 25 Hz, the sample gets pulverized to a size of less than 160 µm (Figure 17, right) and is suitable for analysis such as drug control.
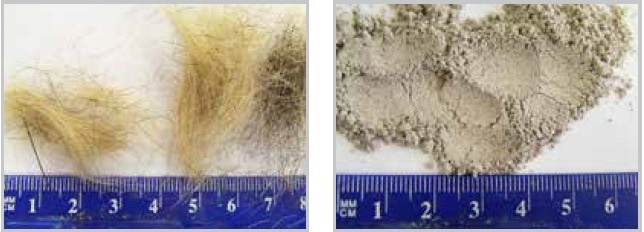
Figure 17. Human hair before and after fine grinding in the MM 400.
Mills from RETSCH are ideally suited to pulverize different types of forensic samples for analysis or extraction of nucleic acid. Moreover, it is possible to easily prepare larger samples such as complete bones in two steps by pre-cutting and fine grinding.
Washing Procedure to Obtain Intact Bacterial Cells from Human Tissue
Infections may occur following artificial replacement of joints such as knees or elbows: One of the major risks associated with joint replacements is the so-called prosthetic joint infection (PJI) brought about by different species of bacteria. In the case of prosthetic joint infections, for instance, it is essential for the bacteria on the tissue samples to be washed off without destroying them, to cultivate them later for diagnostic purposes.
The current reported PJI rates lie in the range of 2%, following a number of improvements with regard to operating rooms, prophylactic antibiotics, surgical techniques, and careful selection of patients among other factors, suggesting that there is still a considerable number of infections each year. PJI may occur within three days following the surgical procedure; however, it can also take years to break out. Since PJI is caused by fairly different microorganisms, it is complicated to perform classification for qualitative therapies.
Unfortunately, based on the technique adopted, the documentation rate is restricted — in some cases, it is not possible to document the infection using conventional methods. The Mixer Mill MM 400 has a role to play here. The procedure is simple: 20 ml of sterile demineralized water is used for diluting the samples, which are placed in an autoclaved steel jar with 5 ml glass beads of 1 mm diameter. Disposable jars that are agitated for 3.5 minutes at 30 Hz may also be used. In this process, the microorganisms are washed off the sample without being destroyed, enabling them to be easily reproduced afterward on an agar plate. A good documentation rate can be achieved using this method (A.-L. Roux et. al 2010)1. Basically, it can be used on any solid infected tissue sample, irrespective of the existence of implanted material.
Mixer Mill MM 400: A True Multi-Purpose Lab Mill
All the application examples discussed above demonstrate that RETSCH’s Mixer Mill MM 400 is highly versatile to use due to a broad selection of accessories. It is best suited for cell disruption in single-use vials, for example, 50 ml conical centrifuge tubes or smaller reaction vials (0.2/1.5/2/5 ml). The MM 400 is ideal for grinding or disruption of a wide range of hard, brittle and soft, fibrous, semi-hard, or elastic materials, such as bones, plants, cell tissue, pine needles, pharmaceutical products, minerals, feathers, wood, or chemicals. Operating with a maximum frequency of 30 Hz, it produces grind sizes down to 5 μm.

Figure 18. Mixer Mill MM 400 with adapter for 8 x 50 ml conical centrifuge tubes (below) and various accessories, for example, adapters for single-use reaction vials (left), grinding jars, and grinding balls (right).
Conclusion
From Bead Beating for cell disruption through homogenization and pulverization to cryogenic grinding, RETSCH offers reliable and convenient solutions for sample preparation in fields such as diagnostics, biotechnology, microbiology, agriculture, and forensics.
References and Further Reading
- A.-L. Roux, V. Sivadon-Tardy, T. Bauer, A. Lortat-Jacob, J.-L. Herrmann, J.-L. Gaillard and M. Rottman: Diagnosis of prosthetic joint infection by beadmill processing of a periprosthetic specimen; Clinical Microbiology and Infection, 2010, European Society of Clinical Microbiology and Infectious Diseases, CMI, 17, 445–450.

This information has been sourced, reviewed and adapted from materials provided by RETSCH GmbH.
For more information on this source, please visit RETSCH GmbH.