Supramolecular chemistry has garnered significant attention in materials science. This is because noncovalent interactions enable the addition of unique behavior to otherwise covalent systems. The most significant attribute is the low-energy barrier between the associated and dissociated state of molecules. This paves the way for accessible reversibility, as discussed in the journal Polymers for Advanced Technologies.

Study: Self-healing behavior in blends of PDMS-based polyurethane ionomers. Image Credit: Photobank.kiev.ua/Shutterstock.com
Ionic interactions offer the highest non-covalent cohesive strength in supramolecular assemblies. In polymer chemistry, noncovalent interactions have been successfully used both as main-chain and side-chain connections for supramolecular polymer networks (SPN).
Despite the high cohesive strength, the lack of directionality and association specificity of ionic interactions is detrimental to a more diffused adoption. Ionomers can bear a single charge type, identifying cationomers and anionomers, or both of them, in so-called zwitterionomers.
This study discusses the creation of a novel poly(urea-urethane) based on a PDMS main chain that exhibits elastomeric behavior at room temperature. Ionic moieties were introduced as side-chain pendants in poly(urea-urethane) siloxane-based polymers to achieve a robust yet flexible elastomer with the ability to recover from a damaged state.
PDMS-Based Poly(Urea-Urethane) Ionomers Synthesis
PDMS-based poly(urea-urethane) synthesis was performed in a two-step process—pre-polymerization and chain-extension. Prepolymerization was performed in the same fashion for both cationomer and anionomer.
The blending of cationomer and anionomer was carried out by hot mechanical mixing. The stoichiometric ratio between equivalents of cationic and anionic functionalities was considered. The prepared cationomeric and anionomeric poly(urea-urethane) samples were named PUU CAT1.5 and PUU ANC1.5, respectively. The resulting ionic blend was named PUU CAT1.5-ANC1.5.
Results
The behavior of the synthesized polymer was consistent with the formation of urethane linkages, with a decrease in isocyanate content. Molecular weights achieved were quite similar between cationomer and anionomer.
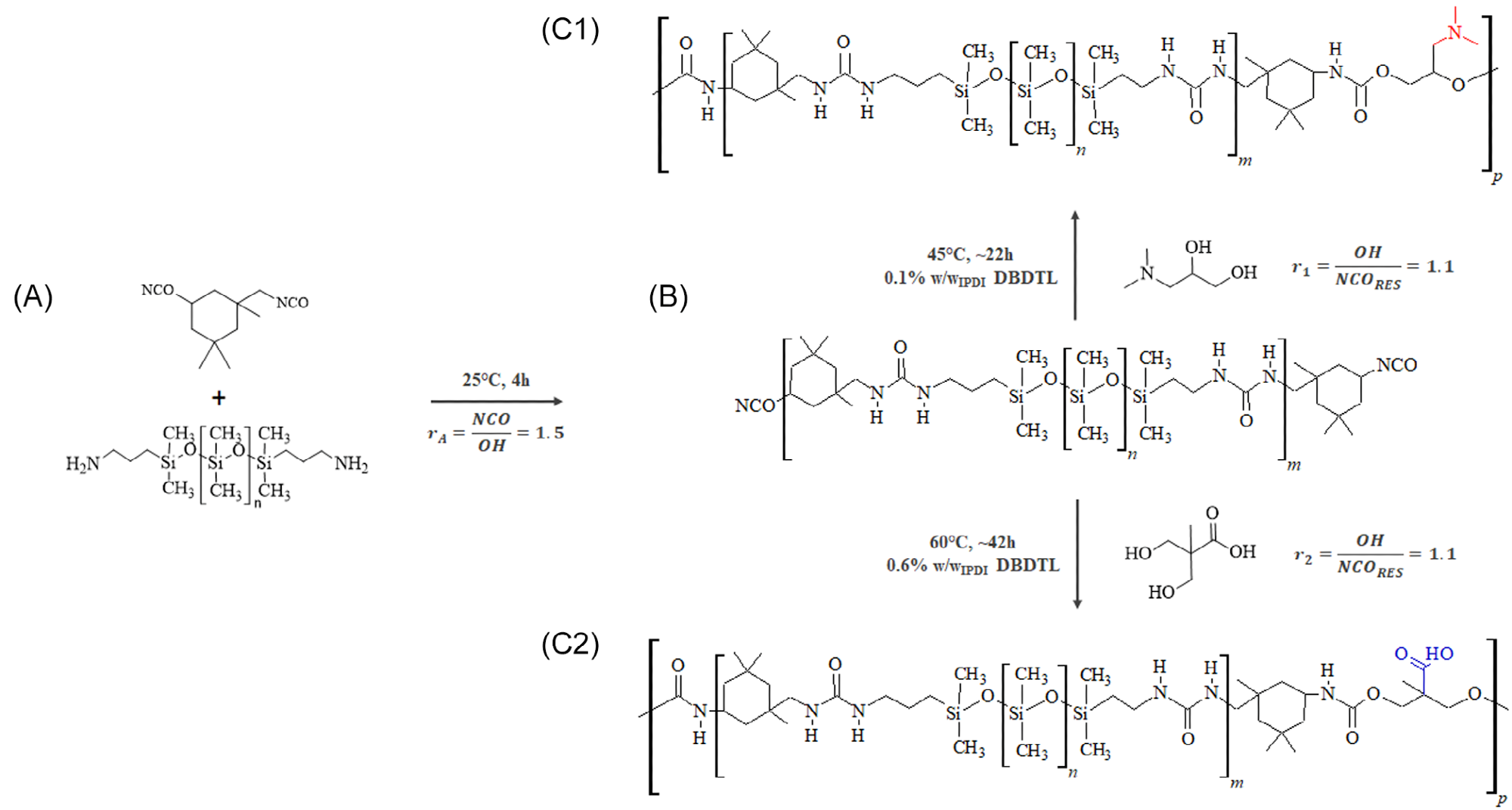
Raw ionomers synthetic path. The monomers IPDI and PDMS-NH2 (A) are combined to form the prepolymer (B). Then, they are chain-extended to give either the cationomer (C1), with tertiary amine functionality (red), or the anionomer (C2), with carboxylic acid functionality (blue). Image Credit: Da Via, et al., 2021. Image Credit: Da Via, F., et al., Polymers for Advanced Technologies
A slightly lower molecular weight in the anionomeric polymer is because of the partial polymerization hindering due to the presence of carboxylic acid functionalities in the chain extender. The material appeared completely homogeneous, colorless, and transparent. Phase segregation between hard urethane and soft PDMS chains was, however, present.
Thermal transitions for ionomers and the blend corresponded to the glass transition of the hard phase, as the IPDI cycloaliphatic structure hinders crystalline conformational organization. Polysiloxane backbone showed great thermal resistance.
Viscoelastic properties were evaluated to detect modifications demonstrating the presence and the effect of ionic interactions. At 25 °C, the blend PUU CAT1.5-ANC1.5 exhibits an elastic shear modulus of 1.41 MPa.
On the other hand, its shear modulus at 75 °C was higher than both ionomers taken singularly, evidencing the reinforcing effect of ionic interactions in supramolecular cohesion. At room temperature, the presence of rigid domains hinders molecular mobility, making less relevant long-range intermolecular interactions.
Tensile tests to assess the mechanical properties of the poly(urea-urethane) blend showed that even for temperatures well below the glass transition, a clear effect was detected due to ionic interactions. This condition was reflected in fracture properties, with higher stress and lower strain at break. The Young modulus values agree with the results from rheological tests as they are intermediate between raw anionomer and cationomer values.
In the case of “soft polymers,” such as the material discussed in this study, structural recovery occurs mainly by interfacial diffusion across cracks. Different sets of healing conditions were considered here for a better understanding of the role played by environmental parameters in healing mechanisms.
The proximity between oppositely charged functionalities increases the intensity of supramolecular interactions, accelerating chain diffusion across the interface and material integrity restoration.
Data for healing efficiency versus healing time shows a significant improvement in tensile strength recovery. Fracture toughness increased even above its starting value as a consequence of the plasticizing effect of the water absorbed from the high humidity environment.
Outcomes
The notable difference between the solubility parameters of PDMS and urea/urethane functionalities induced partial phase separation, leading to a segmented nature and elastomeric behavior.
The blending of the synthesized anionomer and cationomer led to a material that retains the original elastomeric behavior and exhibits enhanced supramolecular cohesion. This microstructure and supramolecular interactions led to a higher transition temperature for the poly(urea-urethane) blend.
The blend also exhibited a more fragile behavior and a significant increase in tensile strength. Oppositely charged functionalities were able to aggregate more extensively into ionic clusters, thus improving the supramolecular cohesion by noncovalent crosslinking. The material also showed hints of self-restoration capabilities at room temperature.
Both humidity and heating allowed to overcome the limitations imposed by restricted molecular mobility on the recovery of pristine properties. The excellent results reported above can help pursue the development of new ionic polyurethane blends with self-healing abilities and to form interpenetrating polymer networks (IPN) based on supramolecular ionic interactions.
Journal Reference
Da Via, F., et al. (2021) Self-healing behavior in blends of PDMS-based polyurethane ionomers. Polymers for Advanced Technologies. https://onlinelibrary.wiley.com/doi/10.1002/pat.5537