AZoM talks to Professor Oren Scherman about his research relating to a novel hydrogel that is able to achieve extreme compressibility under high pressures.
Please can you introduce yourself, tell us about your background within material science, and how you began researching material properties and hydrogels in particular?
When I started my own research group, I was interested in investigating interfacial self-assembly in aqueous media to create functional materials. This led to the seminal report of a self-assembled hydrogel mediated by cucurbiturils (CBs) in 2010. Since this time my group has made several generations of hydrogels for various applications underpinned by fundamental investigations.
What are hydrogels, and what are their most common applications?
Hydrogels are a broad class of materials that are capable of holding large amounts of water within their permanent or transient network. The first example of a hydrogel (named as “hydrophilic gels”, Nature, 1960, 185, 117) was developed for biological use, such as contact lenses. On account of their high-water content, hydrogels have been widely used in tissue engineering, drug delivery, wearable bioelectronics, as well as soft robotics.
Hydrogels are usually soft or ‘jelly-like’; could you explain how this is a result of their chemical structure?
Polymeric hydrogels are often soft as they are composed of polymers (long strands of molecules) that swell and tangle together in water to form a gel. The majority of previous work has focused on using either covalent crosslinks or fast-associative non-covalent crosslinks for the fabrication of hydrogels. By slowing down the time it takes for the crosslinks to fall apart, we improved the mechanical properties of the gels.
What inspired you to try to reverse this and create a hydrogel that was capable of becoming hard/compressible?
Although significant strides have been made in the development of soft and stretchable hydrogels over the last two decades, a major challenge that remained was to achieve extreme compressibility under high compressive loads. We therefore wanted to address this issue and create a new type of hard and compressible hydrogel.
My group has been interested in making gels with emergent properties for many years. Our approach is somewhat unique, as we aim to understand and control the chemistry on the molecular scale. By understanding the material in such a fundamental manner, we start to push the boundaries of how materials behave.
Could you please explain the process of developing your ‘super jelly’?
The development began more than 10 years ago when we reported the very first hydrogel that could be held together using these “barrel-like” CB molecules. Since these first-generation gels, we have made strides towards gels that are biocompatible and soft for use in the treatment of Glioblastoma brain cancer and delivery of therapeutics directly to the brain. We have also been developing these hydrogels towards high-performance materials applications.
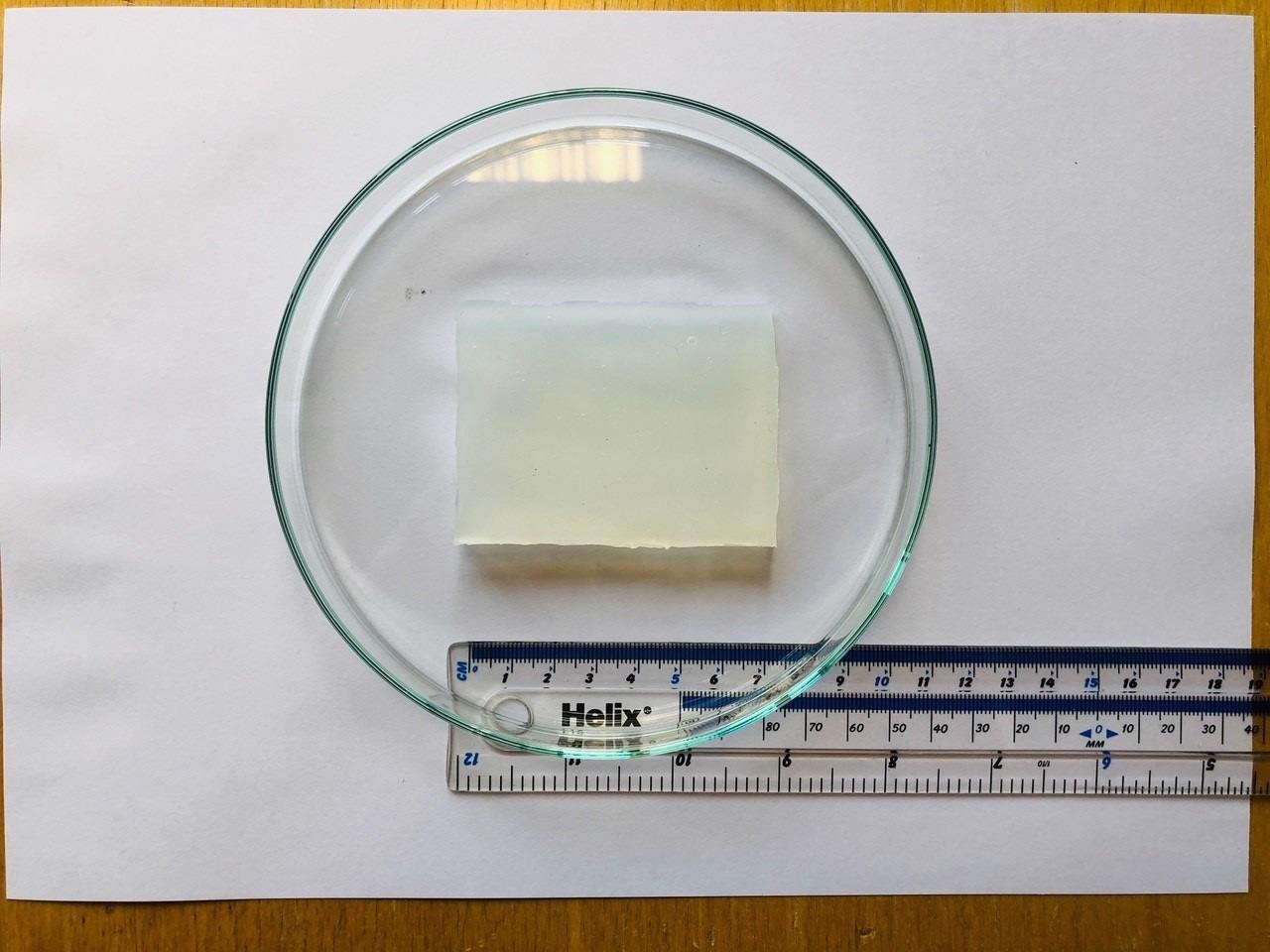
Image Credit: Cambridge University
There are ever-increasing demands on materials to behave in counterintuitive ways and carry out multiple functions. We developed these gels as we were trying to push the boundaries of material properties and function.
Despite your new material being composed of 80% water, it is still able to completely recover to its original shape under immense pressure. What properties enable it to do this and what are some of the other mechanical properties of this new material?
The key to achieving extreme compressibility and rapid self-recovery is the introduction of slow-dissociative non-covalent crosslinks that hold the whole network together across short time scales. This characteristic enables us to access glass-like supramolecular polymeric hydrogels with unprecedented compressive strength up to 100 MPa at 93% strain with room-temperature self-recovery within 2 min. This sets a new benchmark in the area of supramolecular soft materials.
It is stated that the ‘jelly’ can withstand the pressure of a car or the weight of an elephant. Could you explain what prevents the hydrogel from bursting under this enormous pressure?
It is the crosslinks that we have designed and embedded within the network; they fall apart or dissociate much slower than others. They also are able to act as sacrificial bonds to dissipate applied energy such as compression, which in turn prevents the hydrogel from bursting under pressure.
The material has been compared to shatter-proof glass under pressure – why is this? Does it share any properties with this material?
We tested our hydrogels under extreme pressures to see what they could withstand. The value was as high as 1.0 GPa, which is equal to traditional bulk glasses without any water. Even under such high pressure, no fracture was observed. Our glass-like hydrogels also share very similar rheological behavior with glassy polymers at room temperature. This is the first time that hydrogel materials have entered this state.
Your new material could revolutionize the soft robotics sector as well as find uses in many other industries; which sectors do you personally think would benefit most from its diverse properties?
We envision that our glass-like hydrogels could be useful for soft robotics that are designed to operate in extreme working environments with high compression loads. We are also interested in exploring the materials as a cartilage replacement and in a range of bioelectronic applications.

Image Credit: Cambridge University
What is personally the most exciting aspect of this new research to you?
The most exciting part of this new research is that we have gained fundamental insight into how to control the mechanical properties of hydrogels across a broad range of frequencies. This will enable us to design and make the next generation of materials that can have a real societal impact.
What are the next steps for this subject matter? Do you hope to conduct further research around material properties, and hydrogels in particular?
We are now very interested in applying our materials in bioelectronic and biomedical applications. We are collaborating with colleagues to further explore the exciting new potential of our glass-like hydrogel materials.
Where can readers find more information?
Link to the paper: https://www.nature.com/articles/s41563-021-01124-x
Link to youtube: https://www.youtube.com/watch?v=mSyi9pWuTgE
Link to the Melville Laboratory: https://www.ch.cam.ac.uk/group/melville/melville-laboratory-polymer-synthesis
Link to the Scherman Group: https://www.ch.cam.ac.uk/group/scherman
About Professor Oren Scherman
 Oren Scherman is a Professor of Supramolecular and Polymer Chemistry at the University of Cambridge. He is also the Director of the Melville Laboratory for Polymer Synthesis within the Yusuf Hamied Department of Chemistry. Oren started his independent academic career at the University of Cambridge in 2006 and was promoted to reader in 2012, appointed the Director of the Melville in 2013 and full professor in 2015. Prior to Oren’s appointment at Cambridge, he was a postdoc at the Eindhoven University of Technology (TU/e) after his PhD at the California Institute of Technology (Caltech). He first began researching polymers during his Ph.D. which led to a postdoc where his interest in self-assembled supramolecular polymers and assembled materials was heightened.
Oren Scherman is a Professor of Supramolecular and Polymer Chemistry at the University of Cambridge. He is also the Director of the Melville Laboratory for Polymer Synthesis within the Yusuf Hamied Department of Chemistry. Oren started his independent academic career at the University of Cambridge in 2006 and was promoted to reader in 2012, appointed the Director of the Melville in 2013 and full professor in 2015. Prior to Oren’s appointment at Cambridge, he was a postdoc at the Eindhoven University of Technology (TU/e) after his PhD at the California Institute of Technology (Caltech). He first began researching polymers during his Ph.D. which led to a postdoc where his interest in self-assembled supramolecular polymers and assembled materials was heightened.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.