There has been considerable press attention on several fires linked to e-scooters, e-bikes, and smartphones, with several fires starting on commercial flights. The link between these seemingly unconnected events is lithium-ion batteries. This article examines why lithium-ion batteries are prone to failure and how we can explain their popularity despite these risks.

Image Credit: H.E.L Group
The standard electrical cell configuration comprises the basic structure of lithium-ion batteries. These lithium-ion batteries contain a cathode that serves as the source of the lithium ions and will determine the cell’s voltage and capacity, along with a cathode that will accumulate and release the lithium ions. Both elements are submerged in an electrolyte, which permits ion movement and movement of a separator to prevent contact between the cathode and anode.
A very light metal, lithium also has a very high electrochemical potential, which renders it extremely powerful in the design and function of batteries. However, high electrochemical potential means lithium is also highly reactive. Anodes of this metal have a comparatively low melting point (180 oC), and lithium can crystallize under charge-discharge processes. This crystallization forms branched structures called dendrites with high conductivity, which can end in short-circuit events.

Image Credit: H.E.L Group
Lithium-Ion Battery Risks
The main risks of lithium-ion batteries are battery fires, which are a product of thermal runaways.
Thermal runaways is the name given to processes in which the heat released from a chemical reaction accumulates. This heat accumulation accelerates the reaction further and results in a positive feedback loop. Certain electrolytes, if used, can exacerbate this reaction.
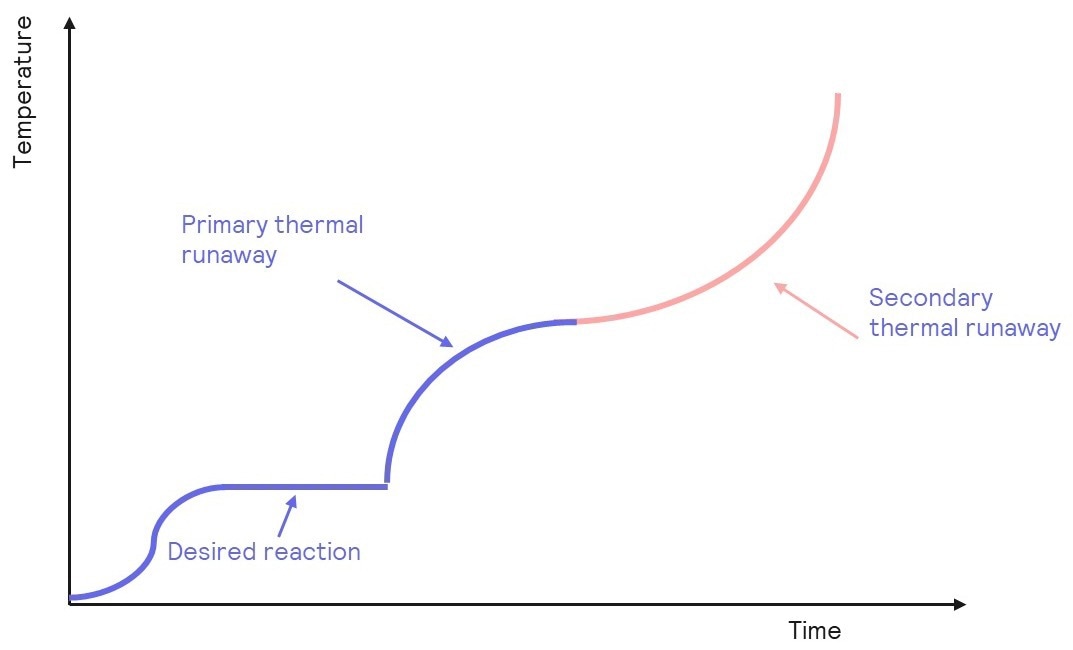
Image Credit: H.E.L Group
The electrolytes typically used in lithium-ion batteries are organic carbonates, e.g. diethyl carbonate or propylene carbonate. These chemicals enable the conductivity to reach levels that permit electrical transport through the dissolution of lithium salts. These compounds are, however, susceptible to thermal decomposition; flammable gases can accumulate inside the battery throughout this process, which, when paired with high temperatures, can result in fire.
Despite these significant drawbacks, lithium-ion batteries offer significant advantages and are still used. Additional measures can be put in place that will result in safer batteries. There are four principal requirements for a battery to be considered safe, these are:
- A stable and controlled solid-electrolyte interface (SEI)
- High conductivity
- The thermal stability of the lithium salt
- The cathode material should not dissolve in the electrolyte
The second and fourth of these requirements are achieved using a combination of lithium salts and a material that is not soluble in the electrolyte.
The first and third requirements invite room for improvement.
Another option is using strategies like temperature-sensitive separators. The separator’s material, in this solution, melts when it attains a specific temperature, increasing the resistance. The extra resistance is typically enough to halt the battery, preventing additional heat production and stopping a thermal runaway event.

Image Credit: H.E.L Group
Safely Using Lithium-Ion Batteries
The study of heat exchange, or calorimetry, is deployed to identify the risks inherent in lithium batteries and establish how to address them. During the development phase, batteries must be put through testing conditions and the potential effects of failure.
Batteries can behave anomalously in these scenarios, particularly in light of heat accumulation, and to prevent accidents from happening, accurate recording is essential. Batteries should be rigorously tested, and the results recorded for batteries to be sold and distributed. These tests must then be compared to the regulations – e.g. EN 60086-4, IEC 62619, or UL 1642.
Determining whether lithium ion-powered devices are safe to use when acquired is fundamental. Reports from December 2022 revealed a recent increase in fires linked to lithium batteries – a 28% increase on a monthly average – and often, fire investigators link these events to damaged, poor quality, or incorrectly-charged devices. Market investigations revealed that at least 59 online listings for e-bike and e-scooter chargers fell below minimum standards. This includes those not containing fuses, which would prevent a fire in the case of a fault event.
It is fundamental to follow the manufacturer’s instructions and ensure that devices are up to standards and marked with the appropriate seal of approval when using devices powered with lithium-ion batteries.

This information has been sourced, reviewed and adapted from materials provided by H.E.L Group.
For more information on this source, please visit H.E.L Group.