In this interview, AZoM speaks to Peter Hatton,Managing Director at Hiden Analytical, about quantitative gas analysis via mass spectrometry. Peter answers questions on some of the misconceptions and pitfalls around partial pressure and total pressure measurement and shows how these are easily avoided. The interview examines how effective calibration procedures combined with Hiden’s QGA and EGAsoft user-friendly software lead to excellent quantitative results for research and process applications.
How can the composition of a gas mixture be determined?
The composition of a gas mixture can be determined using Hiden Analytical Mass spectrometers since they can measure the partial pressures of individual components. The gas composition can be measured either through trend analysis of concentration changes in real time, or through a one-time analysis.
The sum of the partial pressures of the components in a gas mixture is equal to the total pressure within the mass spectrometer chamber. By establishing a simple ratio between the partial pressure of each component and the total pressure, researchers can determine the concentration of each component.
However, this method is unsatisfactory for most applications when considering the measurement accuracy question. The primary reason for this is that the partial pressures reported by the Hiden mass spectrometer (MS) are independent of the total pressure reported by the instrument total pressure gauge. Moreover, the gas species present influence the total pressure gauge, meaning that different compositions yield distinct pressure readings.
To achieve an accurate measurement, it is necessary to utilize the sum of partial pressures measured by the Hiden MS as the total pressure and apply sensitivity factors to account for the behavior of individual gases as they pass through the mass spectrometer system.
The determination of relative sensitivity factors for each component necessitates the implementation of a calibration procedure.
How can the gas composition of a gas mixture be accurately measured?
The first step in the gas analysis process is instrument calibration. This is essential for accurate measurements in various gas analysis applications. Two common examples are the gas composition in a gas stream at atmospheric pressure and the flow rate or total volume of evolved gas from a reaction. To calibrate the instrument, a known calibration standard is necessary.
This involves using gas calibration samples, either gravimetrically mixed gas bottles or mixtures created with calibrated Mass Flow Controllers. These samples contain known concentrations of gases and are certified by reputable suppliers.
During calibration, the gas components are analyzed using the same settings and conditions as in the experiment, with the total of the gas components adding up to 100%.
A calibration factor can be determined by comparing the measured and actual values. This factor is relative to a single gas in the composition, typically the bulk gas, and is expressed as a Relative Sensitivity factor (RS). The RS allows for calculating gas concentrations in different mixtures, as long as the RS is relative to the same gas.
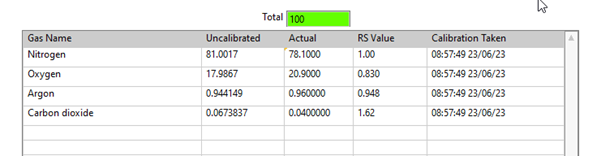
Figure 1. Air Calibration Using QGA software. Image Credit: Hiden Analytical
The calculated RS considers factors such as fractionation in the inlet, ionization, transmission in the quadrupole, and detection efficiency.
Figure 2. Factors effecting RS. Image Credit: Hiden Analytical
It is essential to consider factors like instrument background signals, which can be significant for low-level component analysis. The Hiden MS software can automatically subtract the background signal, ensuring accurate quantification of the product gas.
For gas compositions with wide concentration variations or significant changes, multipoint calibration is employed to achieve the highest accuracy. By utilizing techniques such as background subtraction, spectral deconvolution, and calibration, the instrument can accurately quantify gas composition in terms of a percentage (%), parts per million (PPM), or parts per billion (PPB) ratio.
How do researchers calibrate the instrument for vapor analysis?
The instrument is calibrated for the analysis of vapors similar to gases. However, the production of vapor samples differs. Vapors are not typically available in a gas bottle mixture.
A gas is passed over the liquid to generate known concentrations of vapors to create a vapor stream. The concentration of the saturated vapor stream is determined by the temperature of the liquid using standard data graphs of vapor pressure versus temperature.
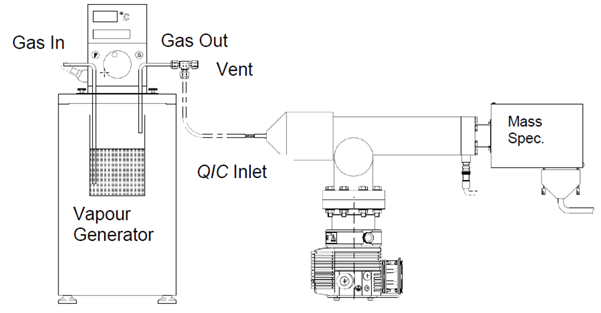
Figure 3. Vapor generator. Image Credit: Hiden Analytical
What are the important factors to control when calibrating a Hiden mass spectrometer for quantitative analysis?
It is necessary to deliver the calibration gas or vapor to the mass spectrometer under the same conditions or as close as possible to the conditions under which the analyte will be sampled.
Users must select a calibration mixture or a mixture with each component concentration midway within the expected concentration range.
For measurements involving a very wide dynamic range, such as from parts per million (PPM) to percentage levels, it is advisable to consider using a multi-point calibration approach.
What tools do Hiden mass spectrometers include to assist users and streamline the calibration routine for everyday use?
The Hiden mass spectrometer sample inlets are suitable for connection to gas calibration streams. This can be achieved either through multi-port valves or simple T-piece connections.
The Hiden QGA software handles all the necessary data calculations for the user. This includes measuring and applying the calibration factors, determining background signal values, performing spectral deconvolution, and presenting the data in both tabular and graphical formats. The data can also be shared with data control centers using OPC tags.
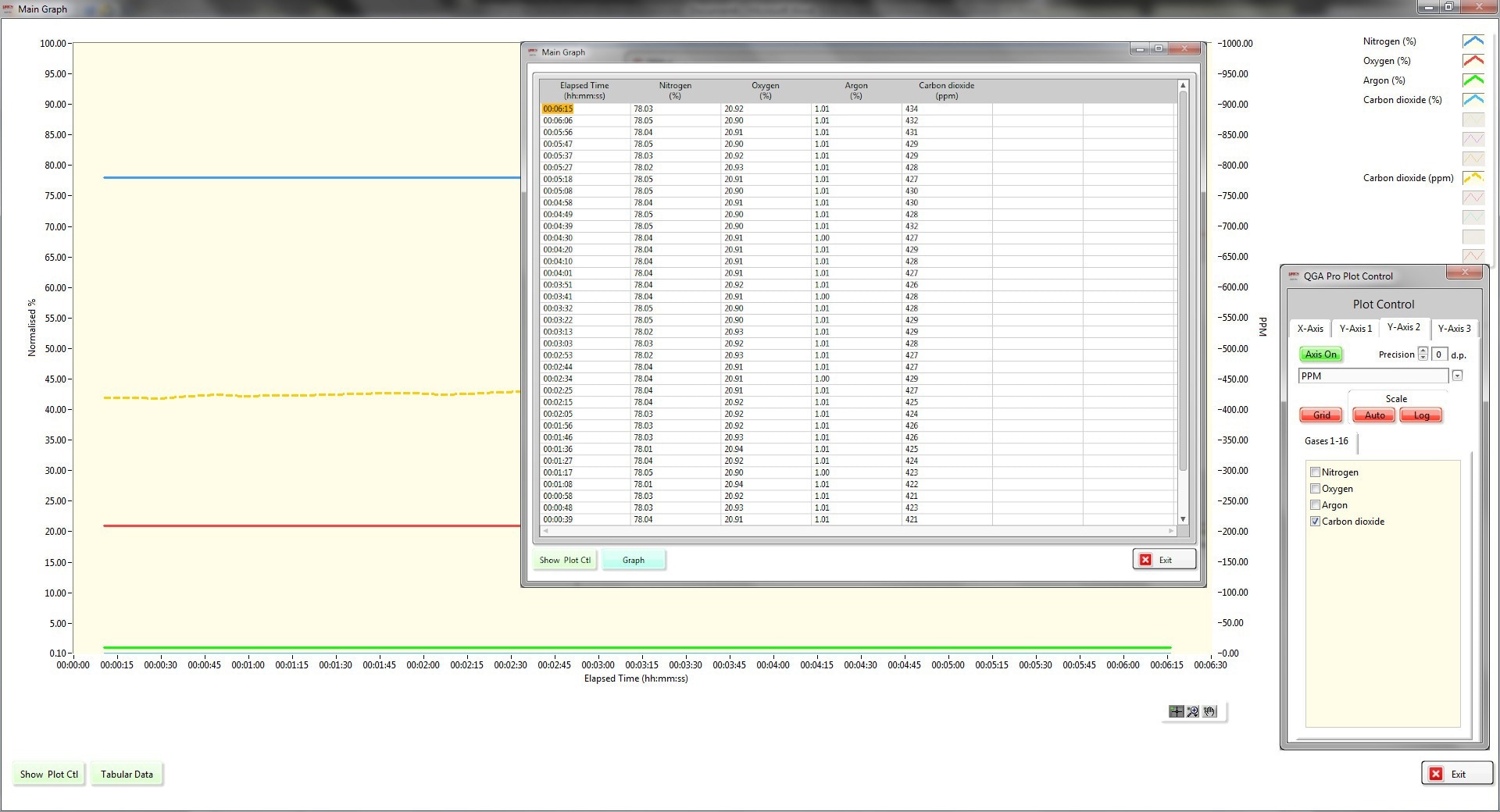
Figure 4. QGA Presentation. Image Credit: Hiden Analytical
How frequently should Hiden mass spectrometers be calibrated to ensure accurate quantitative analysis?
If calibration is performed on a stable system and the conditions and settings remain the same, calibration factors can be applicable for many months. However, it is recommended to perform a weekly check. The QGA software simplifies the calibration process if a new calibration is necessary.
What factors affect instrument calibration?
The instrument can be affected by temperature changes in the mass spectrometer's environment. When utilizing the SEM (secondary electron multiplier) detector, it is necessary to verify the detector's gain periodically.
How can the flow rate or total volume of evolved gas from a reaction be measured?
When the measurement of gas volume evolved during a reaction is necessary, such as in Temperature Programmed Desorption (TPD), TG-MS, or Online Electrochemical MS, an additional step is employed.
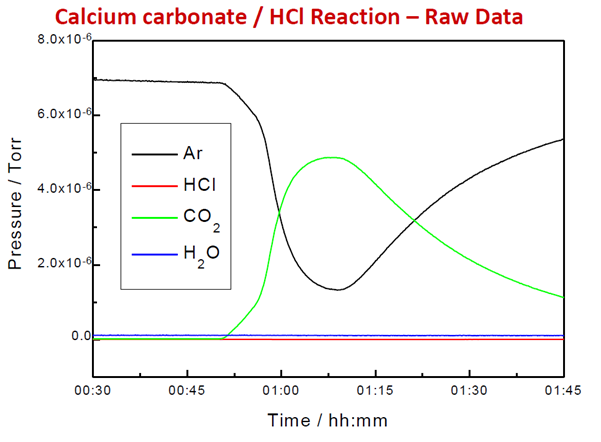
Figure 5. Raw Signal from Evolved Gas in TGA Reaction. Image Credit: Hiden Analytical
A known carrier gas flow rate is passed through the reactor or cell. After subtracting the background and applying calibration factors, the signal of the carrier gas is compared to the signal of the product gases to calculate the flow rate during the experiment.
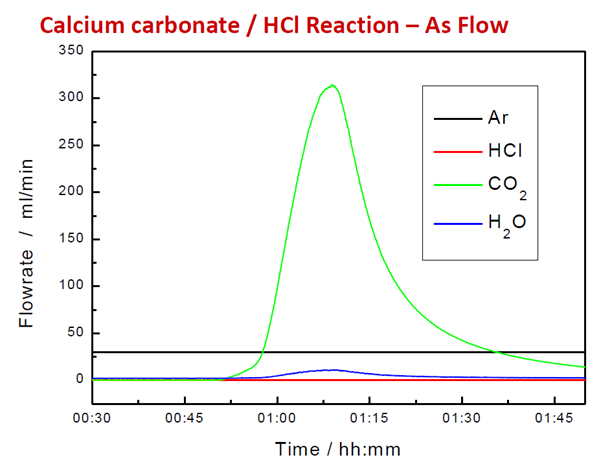
Figure 6. Calculated Flowrate from Corrected Signal. Image Credit: Hiden Analytical
By integrating under the flow rate curve, the total volume of the product gas can be calculated. Hiden's EGAsoft software application facilitates calibration, baseline subtraction, and measurement of evolved gases in TG-MS.
About Peter Hatton

Peter Hatton graduated from a degree in Physics the University of York, UK. He has early-career experience as a project engineer working with solar energy, cryogenics and super conducting magnets.He has been at Hiden Analytical for more than 30 years, working with quadrupole mass spectrometry. He has been the Managing Director of Hiden Analytical since 2008.

This information has been sourced, reviewed and adapted from materials provided by Hiden Analytical.
For more information on this source, please visit Hiden Analytical.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.