Sponsored by Specac LtdReviewed by Maria OsipovaOct 15 2025
Formulation scientists are behind the conversion of active pharmaceutical ingredients (API) into stable, bioavailable, and commercially viable dosage forms, rarely a simple process.

Image Credit: Specac Ltd
Difficulties in formulation development and manufacturing commonly include polymorphic conversions, excipient incompatibilities, API degradation, and real-time process drifts, all of which can jeopardize product safety, efficacy, and regulatory compliance.
The main problem is combining chemical, physical, and performance qualities while assuring batch uniformity and compliance with industry norms.
Spectroscopy has long been used to provide the detailed analytical insights needed to navigate these challenges, but its importance has grown in response to the FDA's Process Analytical Technology (PAT) initiative and the ICH Quality Guidelines, which emphasize Quality by Design (QbD) principles for robust pharmaceutical product and process design.1,2,3
These interconnected needs highlight the importance of quick, adaptable, and practical analytical tools throughout the product life cycle.
Fourier transform infrared (FTIR) spectroscopy meets these objectives by providing sensitive, fast, and non-destructive molecular fingerprinting of solid, semi-solid, and liquid formulations without requiring considerable sample preparation.
It provides inline, at-line, and offline material characterisation in both R&D and manufacturing contexts, offering actionable insights into critical quality attributes (CQAs), expediting process optimization, and assisting with all stages of pharmaceutical formulation development.
This article covers FTIR for pharmaceutical analysis and describes how it promotes formulation success, showcasing a variety of spectroscopy accessories and experimental procedures that could aid R&D leaders, QC managers, and field researchers.
FTIR Spectroscopy – Versatile and Accessible for Formulation Needs
FTIR spectroscopy characterizes compounds based on their ability to absorb infrared (IR) light. Typically, spectra are recorded in the mid-IR range (4,000-400 cm−1); however, some pharmaceutical applications cover the near-IR (12,800-4,000 cm−1).4
A spectral 'fingerprint' is generated, reflecting the vibrational modes of chemical bonds in the sample.
These modes are extremely sensitive to the molecular environment, making FTIR perfect for monitoring polymorphic forms and other minor changes, as well as providing formulation scientists with quick access to comprehensive chemical insights without harming the material.5
FTIR's broad application, from powders and tablets to gels, suspensions, and even multicomponent blends (for example, excipient compatibility testing), makes it an invaluable tool for iterative formulation design and optimization.
Accessories for Real-World Formulation Analysis
In pharmaceutical settings, feasible FTIR analysis depends on accessories that tailor the spectrometer to the desired sample types. Dedicated accessories enable the three primary FTIR sampling modes: transmission/absorbance, attenuated total reflectance (ATR), and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS).
Specac’s introductory guides on transmission vs ATR spectroscopy and DRIFTS provide further details.
For teams dealing with solid, semi-solid, and liquid formats, sampling mode flexibility is critical for streamlining workflows while also guaranteeing robust analytical sensitivity and reproducibility across a wide range of formulation activities. The Specac line includes a variety of accessories to support these workflows.
Simplifying Liquid Analysis
The Specac Pearl™ Accessory makes solution-based formulations easier to prepare. The sample cell allows for quick and easy loading and cleaning while maintaining a precise route length, substantially simplifying transmission FTIR analysis.
A previous application note demonstrated how the Pearl™ was used to analyze two over-the-counter simeticone drugs in distinct doses (tablet and liquid form).
The API content was evaluated using the European Pharmacopoeia standard of toluene extraction, followed by FTIR analysis, and compared to reference solutions (Figure 1). Both test samples closely matched the dosage specified on the label, indicating that the Pearl is an ideal choice for QC labs.
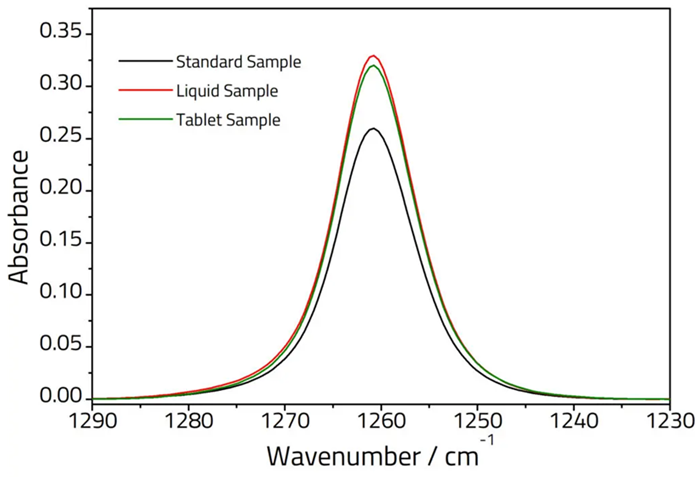
Figure 1. Overlay of FTIR spectra obtained using the Pearl™ for three different simeticone formulations. This spectral region corresponds to the methyl deformation vibration of the API. Image Credit: Specac Ltd
Formulation and QC of Peptide Drugs
The Harrick ConcentratIR2™ Multiple Reflection ATR Accessory is ideal for low-volume liquid samples. Its multi-bounce ATR crystal improves signal-to-noise ratio, even at low analyte concentrations.
A new Application Note shows high-quality spectra of two peptide medicines (ziconotide and calcitonin) in the µg/mL range, directly from injectable formulations with simple buffer subtraction. ConcentratIR2™ is an effective QC and formulation development tool for therapeutic peptides and proteins at low concentrations.
Precision for Solids and High-Stress Conditions
The Specac Golden Gate™ Diamond ATR Accessory is commonly used for formulation research. Its monolithic diamond crystal is highly durable and has great optical clarity, making it perfect for routine analysis of solid and semi-solid dosage forms. The range includes versions for particular applications:
- The Golden Gate High Temperature ATR Accessory allows for temperature ramping and analysis up to 300 °C, which is ideal for polymorph screening and excipient compatibility testing.
- The Golden Gate In-Situ Reaction Cell allows for real-time monitoring of samples at high temperatures and pressures, which is ideal for stress testing and stability research.
Applying FTIR in Pharmaceutical Formulation and Control
FTIR provides rapid, non-destructive molecular insights that aid formulation development and manufacturing throughout the product's lifecycle. Notable application areas include:
Product Formulation Design
Drug-excipient compatibility studies use shifts in important spectral bands to look for undesirable molecular interactions. For example, ATR-FTIR and complementary approaches were used to show that levodopa, an important Parkinson's disease treatment, is incompatible with numerous common excipients.6,7
Polymorph Monitoring
Different polymorphs influence stability and bioavailability, potentially affecting safety and efficacy, although modest IR alterations can identify different forms.8
Researchers used the Golden Gate High Temperature ATR Accessory to unambiguously profile paracetamol polymorphs using variable temperature ATR-FTIR. These phase shifts are critical for commercial manufacturing but difficult to detect using other approaches due to similar transition temperatures.9
Quality Control in Pharmaceutical Manufacturing
Once a formulation has been chosen, maintaining batch uniformity is critical. Blend homogeneity is crucial for all solid oral formulations, particularly APIs with a restricted therapeutic window.10 Moisture content is an important CQA for solid dosage forms, and API identity and concentration must also be accurately assessed.
- Several studies have established NIR-based monitoring approaches for real-time analysis of homogeneity in blending processes, ranging from R&D to manufacturing scales.11-13
- In line with QbD principles, researchers validated an analytical approach for measuring moisture content in pharmaceutical tablets ranging from two to 20 % using DRIFTS data from a handheld NIR spectrometer.14
- DRIFTS provides a non-destructive alternative to standard Karl Fischer titration for analyzing moisture in the chemotherapeutic medication 5-fluorouracil.15
- Pimavanserin, a therapy for Parkinson's disease psychosis, was successfully quantified using a similar manner. Many additional APIs can be measured with FTIR.4, 16
These examples demonstrate why FTIR-enabled analyses are increasingly being included in PAT frameworks and continuous manufacturing methods.
The speed of FTIR data collecting and the flexibility of chemometric models enable real-time monitoring of CQAs and the application of QbD principles, such as detecting process drifts caused by raw material variability.17
Product Integrity: Ensuring Consistency and Compliance
Impurity and identity profiling are critical to product safety. FTIR has applications outside batch variability and release testing, such as:
- Rapidly identifying expired vs compliant co-amoxiclav pills, a common antibiotic. This is assumed to represent the slow development of breakdown products from the less stable clavulanic acid component.18
- Identifying authentic and counterfeit pharmaceutical products. One study used ATR-FTIR fingerprinting (1,800-525 cm−1) to accurately detect counterfeit and genuine tadalafil and sildenafil pills based on composition differences.19
Product Integrity: Ensuring Consistency and Compliance
Looking ahead, two promising prospects in pharmaceutical formulation warrant mention:
1. Point-of-Care Evaluation of 3D Printed Dose Forms
Although levetiracetam is currently the only commercial example, growing acceptance of 3D printed dose forms in personalized medicine is expected in the near future.20,21
- Based on existing results with griseofulvin, indomethacin, and nifedipine formulations, FTIR could improve quality control for 3D printed dosage forms in point-of-care clinical settings.22
2. Potential Use in RNA Therapies
Many RNA therapies are under clinical trials, and the field requires appropriate analytical methodologies for these novel drugs.23, 24
- FTIR can detect RNA structure25 and aid in basic RNA biology research.5 It may provide value to pharmaceutical RNA formulations; however, this remains to be explored.
Product Integrity: Ensuring Consistency and Compliance
Rapid and dependable analytical tools are essential in an industry where product quality, regulatory compliance, and patient safety are paramount. For formulation scientists, FTIR spectroscopy provides speed and variety to help them make better judgments and optimize their efforts throughout the development life cycle.
Its importance is expected to expand as the industry transitions to a modern, data-driven approach to pharmaceutical R&D and manufacturing, based on PAT and QbD frameworks that link risk mitigation to real-time data.
Inline FTIR analysis holds particular potential for monitoring blend homogeneity in powder mixers in order to avoid harmful excessive blending without disrupting the process.10,26 Real-time data integration provides quick input to manufacturing systems, improving compliance and reducing failures.
The Pearl™ and ConcentratIR2™ accessories for liquids, as well as the Golden Gate line for various sample types, increase FTIR's versatility in formulation applications.
References
- Research, C. for D.E. and (2020). PAT - A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. (online) U.S. Food and Drug Administration. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pat-framework-innovative-pharmaceutical-development-manufacturing-and-quality-assurance.
- Thompson, N. (2024). Understanding ICH Guideline Q14. (online) Pharmaceutical Technology. Available at: https://www.pharmaceutical-technology.com/sponsored/understanding-ich-guideline-q14/.
- Yu, L.X., Amidon, G., Khan, M.A., Hoag, S.W., Polli, J., Raju, G.K. and Woodcock, J. (2014). Understanding Pharmaceutical Quality by Design. The AAPS Journal, 16(4), pp.771–783. https://doi.org/10.1208/s12248-014-9598-3.
- Roggo, Y., et al. (2007). A review of near infrared spectroscopy and chemometrics in pharmaceutical technologies. Journal of Pharmaceutical and Biomedical Analysis, (online) 44(3), pp.683–700. https://doi.org/10.1016/j.jpba.2007.03.023.
- Balduzzi, E., et al. (2024). NAIRDB: a database of Fourier transform infrared (FTIR) data for nucleic acids. Nucleic Acids Research, (online) 53(D1), pp.D157–D162. https://doi.org/10.1093/nar/gkae885.
- Bunaciu, A.A., et al. (2024). Fourier transform infrared spectroscopy used in drug excipients compatibility studies. Applied Spectroscopy Reviews, pp.1–19. https://doi.org/10.1080/05704928.2024.2438747.
- Ledeti, I., et al. (2017). Compatibility study between antiparkinsonian drug Levodopa and excipients by FTIR spectroscopy, X-ray diffraction and thermal analysis. Journal of Thermal Analysis and Calorimetry, 130(1), pp.433–441. https://doi.org/10.1007/s10973-017-6393-2.
- Lu, J. and Rohani, S. (2009). Polymorphism and Crystallization of Active Pharmaceutical Ingredients (APIs). Current Medicinal Chemistry, 16(7), pp.884–905. https://doi.org/10.2174/092986709787549299.
- Zimmermann, B. and Baranovic, G. (2011). Thermal analysis of paracetamol polymorphs by FT-IR spectroscopies. Journal of Pharmaceutical and Biomedical Analysis, 54(2), pp.295–302. https://doi.org/10.1016/j.jpba.2010.08.023.
- Talwar, S., et al. (2022). NIR Spectroscopy as an Online PAT Tool for a Narrow Therapeutic Index Drug: Toward a Platform Approach Across Lab and Pilot Scales for Development of a Powder Blending Monitoring Method and Endpoint Determination. The AAPS Journal, 24(6). https://doi.org/10.1208/s12248-022-00748-4.
- Aruna Khanolkar, Patil, B., Viraj Thorat and Samanta, G. (2022). Development of Inline Near-Infrared Spectroscopy Method for Real-Time Monitoring of Blend Uniformity of Direct Compression and Granulation-Based Products at Commercial Scales. AAPS PharmSciTech, 23(7). https://doi.org/10.1208/s12249-022-02392-9.
- Li, Y., et al. (2019). Development of an In-Line Near-Infrared Method for Blend Content Uniformity Assessment in a Tablet Feed Frame. Applied Spectroscopy, 73(9), pp.1028–1040. https://doi.org/10.1177/0003702819842189.
- Shi, Z., et al. (2022). Development of a Near-Infrared Spectroscopy (NIRS)–Based Characterization Approach for Inherent Powder Blend Heterogeneity in Direct Compression Formulations. The AAPS Journal, 25(1). https://doi.org/10.1208/s12248-022-00775-1.
- Patel, A., et al. (2023). Measurement of Moisture Content in Pharmaceutical Tablets by Handheld Near-Infrared Spectrometer: Adopting Quality by Design Approach to Analytical Method Lifecycle Management. Journal of Pharmaceutical and Biomedical Analysis, (online) 229(115381), p.115381. https://doi.org/10.1016/j.jpba.2023.115381.
- Singh, P., et al. (2009). Development and validation of an infrared spectroscopy-based method for the analysis of moisture content in 5-fluorouracil. Drug Testing and Analysis, 1(6), pp.275–278. https://doi.org/10.1002/dta.47.
- Panda, S.S., et al. (2020). Chemometry-assisted Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS) method for quantification of a novel antipsychotic agent in pharmaceuticals: A case study on ICH Q 14 concept. Vibrational Spectroscopy, 107, p.103039. https://doi.org/10.1016/j.vibspec.2020.103039.
- Igne, B., et al. (2014). Effects and Detection of Raw Material Variability on the Performance of Near-Infrared Calibration Models for Pharmaceutical Products. Journal of Pharmaceutical Sciences, 103(2), pp.545–556. https://doi.org/10.1002/jps.23816.
- Foschi, M., Marziale, M. and Biancolillo, A. (2022). Advanced Analytical Approach Based on Combination of FT-IR and Chemometrics for Quality Control of Pharmaceutical Preparations. Pharmaceuticals, 15(6), p.763. https://doi.org/10.3390/ph15060763.
- Ortiz, R.S., et al. (2013). Counterfeit Cialis and Viagra fingerprinting by ATR-FTIR spectroscopy with chemometry: Can the same pharmaceutical powder mixture be used to falsify two medicines? Forensic Science International, 226(1-3), pp.282–289. https://doi.org/10.1016/j.forsciint.2013.01.043.
- Lyousoufi, M., et al. (2023). Development and Bioequivalence of 3D-Printed Medication at the Point-of-Care: Bridging the Gap Toward Personalized Medicine. Clinical Pharmacology and Therapeutics, (online) 113(5), pp.1125–1131. https://doi.org/10.1002/cpt.2870.
- Jørgensen, A.K., Goyanes, A. and Basit, A.W. (2025). Entering New Domains for 3D Printing of Drug Products. PharmTech, (online) 49, pp.24–2724–27. Available at: https://www.pharmtech.com/view/entering-new-domains-3d-printing-drug-products.
- Deon, M., et al. (2022). A critical review of traditional and advanced characterisation tools to drive formulators towards the rational development of 3D printed oral dosage forms. 628, pp.122293–122293. https://doi.org/10.1016/j.ijpharm.2022.122293.
- Curreri, A., et al. (2022). RNA therapeutics in the clinic. Bioengineering & Translational Medicine, 8(1). https://doi.org/10.1002/btm2.10374.
- Demelenne, A., et al. (2021). Analytical techniques currently used in the pharmaceutical industry for the quality control of RNA-based therapeutics and ongoing developments. Journal of Chromatography A, 1651, p.462283. https://doi.org/10.1016/j.chroma.2021.462283.
- Frédéric Geinguenaud, Militello, V. and Véronique Arluison (2020). Application of FTIR Spectroscopy to Analyze RNA Structure. Methods in molecular biology, pp.119–133. https://doi.org/10.1007/978-1-0716-0278-2_10.
- HATTORI, Y. and OTSUKA, M. (2017). ATR/FT-IR and NIR Auto-correlation Spectroscopic Analysis of Powder Blending Uniformity of Low-content Magnesium Stearate and Potato Starch. Analytical Sciences, 33(1), pp.65–68. https://doi.org/10.2116/analsci.33.65.

This information has been sourced, reviewed, and adapted from materials provided by Specac Ltd.
For more information on this source, please visit Specac Ltd.