Researchers from ACS’ Energy & Fuels have reported on innovative heat-based fabrication techniques that convert PET into supercapacitor electrodes and separator films for repurposed energy storage devices. The fully plastic supercapacitor created from discarded water bottles surpassed the performance of a comparable model that utilized a conventional glass fiber separator.
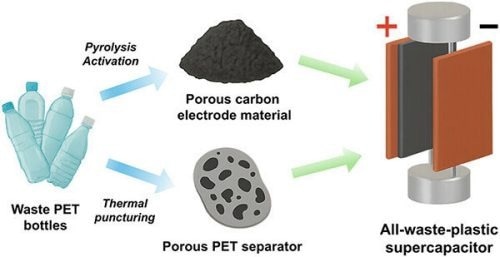 To keep single-use plastics out of landfills, researchers developed a heat-based strategy to upcycle waste PET bottles into high-performance supercapacitor components. Image Credit: Adapted from Energy & Fuels 2025, DOI: 10.1021/acs.energyfuels.5c03370
To keep single-use plastics out of landfills, researchers developed a heat-based strategy to upcycle waste PET bottles into high-performance supercapacitor components. Image Credit: Adapted from Energy & Fuels 2025, DOI: 10.1021/acs.energyfuels.5c03370
A significant number of single-use water bottles made of poly(ethylene terephthalate) (PET) are discarded in landfills; however, there is an increasing interest in upcycling them.
PET is used to produce over 500 billion single-use beverage bottles each year, which generates a significant amount of plastic waste and poses a major environmental challenge. PET-derived supercapacitors hold great potential for diverse applications in transportation and automotive systems, electronics and consumer devices, as well as industrial and specialized sectors.
Yun Hang Hu, Study Lead Researcher, Michigan Technological University
Transforming waste plastics such as PET into carbon-based materials, particularly those that exhibit electrical conductivity, presents an appealing method for producing more economical and sustainable energy storage solutions like supercapacitors.
These devices utilize highly conductive carbon electrodes to efficiently store and discharge substantial amounts of energy rapidly and repetitively.
Consequently, Hu and team aimed to repurpose discarded water bottles into components for a specific type of supercapacitor known as an electrical double-layer capacitor (EDLC).
This device is defined by two porous carbon-based electrodes that are separated by a thin, perforated film submerged in a liquid electrolyte.
Hu's team created two methods to convert used PET water bottles into parts for the upcycled device.
The researchers sliced the plastic bottles into small grains resembling couscous to create the electrodes. The team incorporated calcium hydroxide and subjected the mixture to a temperature of nearly 1300 ºF (700 ºC) within a vacuum environment. This procedure transformed the plastic into a porous, electrically conductive carbon powder. Subsequently, the researchers mixed the carbon powder with carbon black and a polymer binder, which they then dried into thin layers.
For the separator, the researchers flattened small pieces of plastic, approximately the size of postage stamps, and used heated needles to create holes in them. The arrangement of these holes was designed to enhance the flow of current through the electrolyte.
The researchers immersed two porous carbon electrodes in a potassium hydroxide electrolyte solution and positioned them apart using the perforated PET film to construct the PET-based supercapacitor. The upcycled supercapacitor maintained 79 % of its capacitance (storage capacity), whereas a comparable device utilizing a glass fiber separator preserved 78 %.
Hu and team believe this research presents a viable approach for converting PET waste into components for supercapacitors, "opening new opportunities for circular energy storage technologies." The team indicates that the upcycled EDLC is more cost-effective than devices constructed with glass fiber and is entirely recyclable.
With further optimization, PET-derived supercapacitors might realistically transition from laboratory prototypes to market-ready devices within the next five to 10 years, especially as demand grows for sustainable, recyclable energy storage technologies.
Yun Hang Hu, Study Lead Researcher, Michigan Technological University
The authors received financial support from the Charles and Carroll McArthur Fund.
Journal Reference:
Chen, S., & Hang Hu, Y., et al. (2025) All-Plastic Supercapacitors from Poly(ethylene terephthalate) Waste. Energy & Fuels. doi.org/10.1021/acs.energyfuels.5c03370