One of the most important characteristics in determining wine quality is acidity. Acidity is what gives wine its tangy and sour flavor, complementing foods in a palate-cleansing and refreshing way.
Depending on age and type, wines have an acidic pH ranging from 2.5 to 4.5. The typical permissible acid concentration is between 0.5 and 10 g/L.
There are a variety of acids present in wine, the most common of which are tartaric and malic acids. Their quantities vary depending on the grape variety, but tartaric acid is the most common and hence regarded as equivalent to the wine's total acidity.
Unripe grapes are sour because they contain a high concentration of tartaric acid. When grapes ripen, much of the acid is converted to sugar. During the fermentation process, yeasts convert these carbohydrates into ethanol and CO2.
As a result, acidity is an essential criterion when determining wine quality.

Image Credit: Mettler-Toledo - Titration
Discussion
This method was created to determine the tartaric acid concentration of wine via titration with NaOH titrant. The titration is monitored using potentiometric measurement with a pH sensor and ends at pH 7.
Sample
Wine (red), 50 mL
Preparation Procedure
- Calibrate the pH sensor with METTLER TOLEDO buffers 4.01 and 9.21.
- First conduct the titer determination for the titrant, followed by the content determination of tartaric acid in a standard.
- Analysis is performed using 50 mL of wine sample and 0.4-0.5 g of tartaric acid sample.
Chemistry
H2C4H4O6 + 2NaOH → Na2C4H4O6 + 2H2O
Compound
- Tartaric acid, M = 150.09 g/mol; z = 2
Chemicals
- Deionized water
- Standard: Potassium hydrogen phthalate, 0.7 – 1.20 g
- Tartaric Acid
- MT-EU Buffers for calibration of pH sensors (pH 4.01 and 9.21).
Titrant
- Sodium hydroxide, NaOH, c(NaOH) =1.0 mol/L
Instruments and Accessories
- Easy pH (30060041) or Easy Pro (30060044)
- pH sensor, EG11-BNC (30043103)
- EasyPlus Burette 20 mL (30043901)
- Magnetic Stir bar (51191159)
- EasyDirect™ titration software (30065449)
- Titration beakers PP 100 mL (101974)
Method
Source: Mettler-Toledo - Titration
| . |
. |
| EQP /EP |
EP |
| Titration type |
Direct |
| Sample ID |
Tartaric acid content |
| Prestir duration |
20 s |
| Sample size entry |
Fixed Volume |
| Multiple determination |
Yes |
| Endpoint value |
7.0 pH |
| Control |
Normal |
| Stir |
Medium |
| Predispense |
0 mL |
| Calculation |
Content [g/L]; [%] |
| Report |
long |
Results
Source: Mettler-Toledo - Titration
|
[g/L] |
[%] |
| 1 |
6.23 |
0.62 |
| 2 |
6.25 |
0.62 |
| 3 |
6.24 |
0.62 |
| 4 |
6.24 |
0.62 |
| 5 |
6.33 |
0.62 |
| STATISTICS |
|
|
| Mean |
6.25 |
0.62 |
| s |
0.041 |
0.004 |
| srel [%] |
0.65 |
0.72 |
Titration Curve
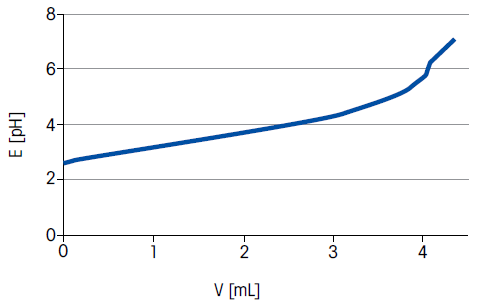
Figure 1. Titration curve for wine. Image Credit: Mettler-Toledo - Titration
Waste Disposal and Safety Measures
The solution must be neutralized prior to final disposal.
Remarks
- The sensor must be cleaned entirely after each analysis.
- Calibrate the sensor before titration.
- Use Ascarite (II) adsorbent to shield NaOH from ambient CO2.

This information has been sourced, reviewed, and adapted from materials provided by Mettler-Toledo - Titration.
For more information on this source, please visit Mettler-Toledo - Titration.