Volumetric Karl Fischer (KF) titration is one method for quickly and accurately determining water content across numerous samples. Based on the reaction of water with iodine, sulfur dioxide, a base, and a short-chain alcohol, which typically acts as the reaction’s solvent, the KF approach works for solids, liquids, and gases.
Any sample works as long as the water inside the sample can be expelled from the sample matrix to then undergo titration by the KF reagent. However, not all solid or semi-solid samples can be easily dissolved in the KF solvent. Some samples require many minutes before they can completely dissolve, or, as in the case of nonpolar substances, they may have solubility that is very limited. Often, co-solvents are added to the KF solvent to change its polarity and dissolution characteristics.
Dissolution can take a significant amount of time, and the number of measurable samples is usually extremely limited. At the same time, it is possible to dissolve these samples externally in an effective solvent and to determine how the resulting solution’s water content is calculated.
This can significantly reduce the time needed for analysis, as there is no waiting time between the measurements until the samples have been dissolved in the KF solution.
This article explores the external dissolution process and clearly explains how a METTLER-TOLEDO EVA V3 KF titrator can precisely calculate butter’s water content.

Image Credit: Mettler-Toledo - Titration
Introduction
Dissolving solid or semi-solid samples into the KF solvent is not always possible. In these scenarios, co-solvents are typically administered to the KF solvent (typically methanol). Formamide is often used for sugars or gelatin-based items such as candies. Chloroform, 1-decanol, or toluene are typically used for nonpolar materials such as oils, grease, wax, and beyond.
However, such solubility is typically still relatively constrained, and dissolution can take an extremely long time. Dissolving the sample in a proper solvent externally and calculating the resulting solution’s water content enables sample analysis that would otherwise be difficult. It also considerably accelerates the analysis, as there is no waiting period after every additional sample until it has completely dissolved in the KF solvent.
In this use case, the butter’s water content is calculated using a METTLER TOLEDO EVA V3 Karl Fischer titrator using the external dissolution approach. The butter is first dissolved in 1-decanol, and the resulting solution is titrated with a 5.0 mg/mL one-component KF titrant.

Figure 1. EVA V3 Karl Fischer Titrator assembly. Image Credit: Mettler-Toledo - Titration
Procedures
KF Concentration:
It is recommended to determine the titrant concentration once a day, using a certified water standard, before evaluating any samples. A 10.0 mg/g water standard is assessed thrice using the KF Concentration method template T012.
Titration:
To calculate the butter’s water content, the following parameters need to be obtained:
- The dissolution solvent’s mass (msol)
- The dissolution solvent’s water content (Blank B)
- Mass of the sample dissolved externally (mext)
- Mass of the sample solution administered into the KF cell (m)
Using these parameters, it is possible to calculate the water content R of the sample (in %) using the following formula:
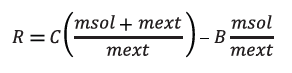
Where C is the water content (in %) of the sample solution given by the formula:
C = (VEQ*CONC-TIME*DRIFT/1000)*(0.1/m)
and B is the blank value (in %), which is determined prior to the determination of the sample solution.
The full procedure consists of the following steps:
- Prepare a couple of clean flasks (or bottles, vials, etc.) that can be closed with a septum or stopper, as well as a magnetic stir bar.
- Label one of the flasks as “Sample” and tare it on the balance. After this, add around two grams of butter to the flask and write down its exact weight as mext.
- Tare the flask again and add around 50 mL of solvent. Write down the added solvent’s weight as msol.
- Put in the stir bar and seal the flask.
- Label the second flask with “Blank,” add roughly the same quantity of solvent, and then seal it. At this point, it is not important to calculate the exact weight, as it is not used for calculation purposes.
- Stir the sample solution on a stir plate until the butter dissolves completely.
- Create a task from the external dissolution method template T013 and then choose a task name, number of samples, and number of blanks.
- Type in the previously recorded mass, msol, into the field ‘Solvent weight’ and mext into the field denoted ‘Extr. sample weight’.
- Begin the task and wait for the KF cell to be ready.
- While waiting, clean the moisture adhered to the inner walls of a 10 mL syringe: Take out around 3.0 mL of solvent from the Blank flask into the syringe, and pull the plunger back all the way. Spin the solvent around the inside of the syringe for some seconds, then place it in the solvent waste. Replenish the syringe with 10 mL of solvent from the Blank flask.
- Once the cell is ready, continue with the blank measurements by administering one to three milliliters of solvent according to the water content. The injected solvent’s mass is calculated via the back weighing approach.
- Finally, calculate the sample solution’s water content by repeating steps 10 and 11.
- The butter’s water content is determined automatically and provided as the main result.
Chemistry
ROH + SO2 + 3 RN + I2 + H2O → (RNH)SO4R + 2 (RNH)I
Solutions and Reagents
- Titrant: HYDRANAL™ Composite 5, c = 5 mg H2O/mL, one-component KF titrant
- Solvents: HYDRANAL™ Methanol dry, 1-Decanol, CH3(CH2)9OH, CAS 112-30-1
- Standard: HYDRANAL™ Water standard 10.0, water content: 10.0 mg/g = 1 %
- Sample: Butter was bought from a local grocery store.
Instruments and Accessories
- Karl Fischer Titrator EVA V3 (30869282)
- Analytical Balance, e.g., XPR205 (30355411)
- 10 mL syringes (00071482)
- 2 sealable vessels (bottles, flasks, vials, etc.)
- Magnetic stir bar

Figure 2. Example of two dissolution flasks. Left: blank flask containing 1-decanol. Right: Sample flask with the butter dissolved in 1-decanol. Image Credit: Mettler-Toledo - Titration
Results
Table 1. Water content of the solvent blank. Source: Mettler-Toledo - Titration
| |
Water content Blank [%] |
Blank size [g] |
| 1 |
0.0426 |
1.6405 |
| 2 |
0.0426 |
1.7030 |
| 3 |
0.0435 |
1.5249 |
| mean |
0.0429 |
|
| s |
0.0005 |
|
| srel |
1.2112 |
|
Table 2. Water content of the butter sample. Source: Mettler-Toledo - Titration
| |
Water content Sample [%] |
Sample size [g] |
| 1 |
13.951 |
0.8130 |
| 2 |
13.825 |
0.8321 |
| 3 |
13.845 |
0.8149 |
| 4 |
13.845 |
0.7496 |
| 5 |
13.820 |
0.8535 |
| 6 |
13.927 |
0.7864 |
| mean |
13.869 |
|
| s |
0.056 |
|
| srel |
0.402 |
|
Both measurement series demonstrated high repeatability with lower relative standard deviations, and the assessed water contents were within expectation.
The EU regulation No 1308/2013 of the European Parliament defines butter as: “The product with a milk-fat content of not less than 80 % but less than 90 %, a maximum water content of 16 % and a maximum dry non-fat milk-material content of 2 %.”1
With a water content of 13.869 %, the measured product accords with EU regulations and may thus be sold in the European Union as butter.
Remarks
This article uses the standard external dissolution template ‘KF vol ext. dissolution (%)’ available on the instrument. As a result, no additional approach is available with this article. Completely dissolving 2 grams of butter in 1-decanol demands around 15 minutes of stirring.
This process can be reduced to around three minutes by subtly heating the solution on a hot plate to 50 °C. While 1-decanol is primarily used as the solvent for dissolution, other nonpolar solvents, such as 1-octanol or chloroform, can also be used. These have similar dissolution characteristics and generate equivalent outcomes.
Waste Disposal and Safety Measures
When working with chemicals, always wear safety goggles, a lab coat, and gloves. Karl Fischer waste should be disposed of as organic solvent waste.
Measured Values
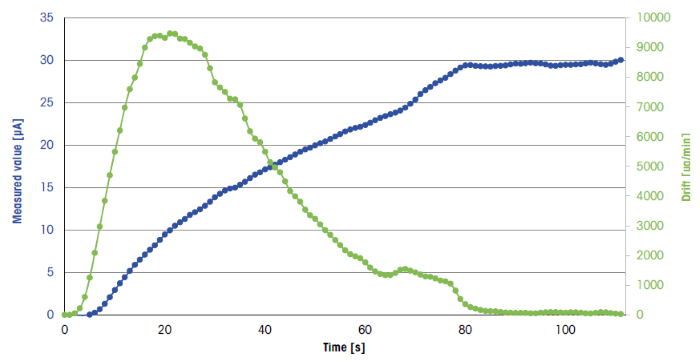
Figure 3. Typical titration curve, taken from the first measurement of the sample solution. Image Credit: Mettler-Toledo - Titration
Table 3. Excerpt of the titration data for the for the first measurement of the sample solution. Source: Mettler-Toledo - Titration
| Time [s] |
Volume [mL] |
Measured value [μA] |
Water [μg] |
Drift [μg/min] |
| 0 |
0 |
-0.038317 |
0 |
0 |
| 1 |
0.00007 |
-0.038603 |
0.351 |
2.3 |
| 2 |
0.00125 |
-0.038813 |
6.271 |
52.8 |
| 3 |
0.00417 |
-0.038992 |
20.921 |
224.8 |
| 4 |
0.01066 |
-0.037040 |
53.481 |
604.0 |
| 5 |
0.02118 |
0.026106 |
106.260 |
1254.5 |
| 6 |
0.03486 |
0.248312 |
174.893 |
2088.7 |
| 7 |
0.05149 |
0.674041 |
258.325 |
2972.9 |
| 8 |
0.07069 |
1.315438 |
354.652 |
3840.6 |
| 9 |
0.09285 |
2.086121 |
465.828 |
4698.8 |
| … |
… |
… |
… |
… |
| 101 |
1.17234 |
29.441185 |
5881.630 |
85.4 |
| 102 |
1.17253 |
29.499565 |
5882.583 |
83.9 |
| 103 |
1.17265 |
29.500348 |
5883.185 |
71.9 |
| 104 |
1.17270 |
29.608127 |
5883.436 |
56.9 |
| 105 |
1.17302 |
29.693978 |
5885.041 |
49.3 |
| 106 |
1.17351 |
29.609366 |
5887.500 |
69.1 |
| 107 |
1.17383 |
29.511491 |
5889.105 |
91.6 |
| 108 |
1.17389 |
29.452495 |
5889.406 |
86.1 |
| 109 |
1.17389 |
29.549945 |
5889.406 |
62.6 |
| 110 |
1.17391 |
29.800828 |
5889.506 |
39.2 |
Method – External Dissolution
General Settings. Source: Mettler-Toledo - Titration
| . |
. |
| Name |
KF vol ext. dissolution (%) |
| ID |
T013 |
| Compatibility |
Titration |
| Method type |
KF Vol External Extraction |
| SOP |
NO |
| Task comment |
NO |
Configuration. Source: Mettler-Toledo - Titration
| Analysis |
|
| Analyze more than one sample |
YES |
| Initial sequence |
NO |
| Final sequence |
NO |
| Open series |
NO |
| Analysis start |
Manual |
| Number of samples |
Six |
| Create statistics |
YES |
| Group samples for statistics |
NO |
| Activate ‘KF conditioning’ after method ends |
YES |
| KF cell |
KF cell 1 |
| Category |
Volumetric (large) |
| Unit for drift |
μg/min |
| Work with solvent exchange |
YES |
| KF pump |
dPump KF 1 |
| Start criteria |
Absolute drift values |
| Min. start drift |
0.0 μg/min |
| Max. start drift |
25 μg/min |
| Blank |
|
| Blank measurement |
Always |
| Open series |
NO |
| Number of blanks |
Three |
| Create statistics |
YES |
| Blank calculation (mean value) |
R2 |
| Blank “B” |
Blank value 1 |
| Unit “B” |
% |
| Result limits |
NO |
| Live View |
|
| Displayed results (Sample) |
1 |
| Field 1 |
R1 |
| Displayed results (Blank) |
1 |
| Field 1 |
R2 |
| Analysis graph: Horizontal axis |
Time |
| Analysis graph: Vertical axis |
Measured value |
| Additional curve |
Drift |
| Conditioning graph: Horizontal axis |
Time |
| Conditioning graph: Vertical axis |
Drift |
Blank Sequence. Source: Mettler-Toledo - Titration
| 1 Drift (Determination – online) |
|
|
| Drift determination |
|
YES |
| Determine online (during conditioning) |
|
YES |
| 2 Blank (Addition) |
|
|
| Prompt for blank addition |
|
YES |
| Blank detection |
|
No |
| Prompt for blank size |
|
YES |
| 3 Titration (KF Vol) |
|
|
| Resources |
|
|
| Titrant |
Titrant |
Titrant 1 |
|
Nominal concentration |
5 mg/mL |
| Sensor |
Sensor |
dSens M143 |
|
Category |
Polarized |
| Stirrer |
Stirrer |
Stirrer 1 |
|
Category |
Magnetic |
|
Stir speed |
35 % |
| Titration |
|
|
| Preparation |
Stir before titration |
10 seconds |
| Control |
Control focus |
Accuracy |
|
Indication |
Amperometric |
|
Unit |
μA |
|
Potential (Upol) |
100 mV |
|
Set current |
30 μA |
|
Cautious mode |
YES |
| Termination |
Type |
Drift stop relative |
|
Drift relative |
25.0 μg/min |
|
Delay |
0 seconds |
|
Min. time |
0 seconds |
|
Max. time |
∞ seconds |
|
At Vmax |
10 mL |
| 4 Result R2: Blank value |
|
|
| Formula type |
Fixed |
|
| Result name |
Blank value |
|
| Formula |
(VEQ*CONC-TIME*DRIFT/1000)*(0.1/m) |
| Unit |
% |
|
| Decimal places |
Four |
|
| Result limits |
NO |
|
Sample Sequence. Source: Mettler-Toledo - Titration
| 1 Drift (Determination – online) |
|
|
| Drift determination |
|
YES |
| Determine online (during conditioning) |
|
YES |
| 2 Sample (Addition) |
|
|
| Prompt for sample addition |
|
YES |
| Sample detection |
|
No |
| Prompt for sample size |
|
YES |
| Prompt for external extraction weight |
|
NO |
| 3 Titration (KF Vol) |
|
|
| Resources |
|
|
| Titrant |
Titrant |
Titrant 1 |
|
Nominal concentration |
5 mg/mL |
| Sensor |
Sensor |
dSens M143 |
|
Category |
Polarized |
| Stirrer |
Stirrer |
Stirrer 1 |
|
Category |
Magnetic |
|
Stir speed |
35 % |
| Titration |
|
|
| Preparation |
Stir before titration |
10 seconds |
| Control |
Control focus |
Accuracy |
|
Indication |
Amperometric |
|
Unit |
μA |
|
Potential (Upol) |
100 mV |
|
Set current |
30 μA |
|
Cautious mode |
YES |
| Termination |
Type |
Drift stop relative |
|
Drift relative |
25 μg/min |
|
Delay |
0 seconds |
|
Min. time |
0 seconds |
|
Max. time |
∞ seconds |
|
At Vmax |
10 mL |
| 4 Result R1: External extraction |
|
|
| Formula type |
Fixed |
|
| Result name |
External extraction |
|
| Formula |
(VEQ*CONC-TIME*DRIFT/1000)*(0.1/m)*((msol+mext)/mext)-B*msol/mext |
| Unit |
% |
|
| Decimal places |
Three |
|
| Result limits |
NO |
|
References and Further Reading
- European Union. (2024). Regulation - 1308/2013 - EN - EUR-Lex. (online) Available at: https://eur-lex.europa.eu/eli/reg/2013/1308/oj/eng.

This information has been sourced, reviewed, and adapted from materials provided by Mettler-Toledo - Titration.
For more information on this source, please visit Mettler-Toledo - Titration.