Researchers at KAIST have achieved a significant extension of battery life purely through the design of the electrode surface.
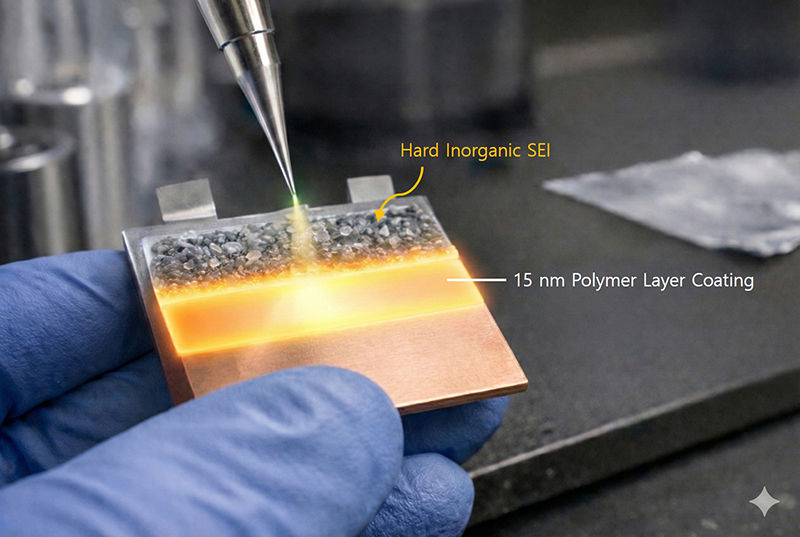 Schematic of an ultrathin artificial polymer layer (15 nm thick) introduced onto the electrode surface. Image Credit: Korea Advanced Institute of Science and Technology
Schematic of an ultrathin artificial polymer layer (15 nm thick) introduced onto the electrode surface. Image Credit: Korea Advanced Institute of Science and Technology
Anode-free lithium metal batteries, which have garnered interest as potential options for electric vehicles, drones, and advanced high-performance batteries, provide significantly greater energy density compared to traditional lithium-ion batteries. Their limited lifespan has posed challenges for commercialization.
KAIST presented their solution to interfacial instability – the primary drawback of anode-free lithium metal batteries – by applying an ultrathin artificial polymer layer measuring 15 nm in thickness to the electrode surface. The study was published in Joule.
Anode-free lithium metal batteries feature a straightforward structure that uses a copper current collector solely in place of graphite or lithium metal at the anode. This configuration provides benefits such as a 30-50 % increase in energy density compared to traditional lithium-ion batteries, reduced manufacturing expenses, and streamlined processes.
Nevertheless, during the initial charging phase, lithium accumulates directly on the copper surface, swiftly depleting the electrolyte and creating an unstable solid electrolyte interphase (SEI), which results in a significant decrease in battery lifespan.
Instead of altering the electrolyte composition, the research team opted for a strategy that involves redesigning the electrode surface where the issue arises. By applying a uniform ultrathin polymer layer on the copper current collector through an iCVD (initiated chemical vapor deposition) process, they discovered that this layer manages interactions with the electrolyte, effectively regulating lithium-ion transport and the pathways for electrolyte decomposition.
In traditional batteries, the solvents in the electrolyte decompose, leading to the formation of soft and unstable organic solid-electrolyte interphase (SEI) layers. This leads to uneven lithium deposition and encourages the development of sharp, needle-like dendrites.
The polymer layer introduced in this research does not easily blend with the electrolyte solvent, resulting in the breakdown of salt components rather than the solvents. Consequently, a robust and stable inorganic SEI is established, which effectively reduces electrolyte consumption and curtails excessive SEI growth.
Through the use of operando Raman spectroscopy and molecular dynamics (MD) simulations, the researchers elucidated the mechanism by which an anion-rich environment develops at the electrode surface during battery operation, resulting in the creation of a stable inorganic SEI.
This innovative technology requires only the application of a thin surface layer, without modifying the electrolyte composition, ensuring high compatibility with current manufacturing processes and incurring minimal cost implications. The initiated chemical vapor deposition (iCVD) process enables large-area, continuous roll-to-roll production, making it suitable for industrial-scale mass production beyond laboratory settings.
Beyond developing new materials, this study is significant in that it presents a design principle showing how electrolyte reactions and interfacial stability can be controlled through electrode surface engineering.
Jinwoo Lee, Professor, KAIST
“This technology can accelerate the commercialization of anode-free lithium metal batteries in next-generation high-energy battery markets such as electric vehicles and energy storage systems (ESS),” added Professor Jinwoo Lee.
Journal Reference:
Lee, J., et al. (2026) A strategic tuning of interfacial Li+ solvation with ultrathin polymer layers for anode-free lithium metal batteries. Joule. DOI: 10.1016/j.joule.2025.102226.