SABIC Innovative Plastics today announced third-party test results that validate the multiple sterilization capabilities of its remarkably tough Ultem* HU1004 polyetherimide (PEI) resin. This high-performance resin - previously utilized for other techniques and now tested for use with the STERRAD NX low-temperature hydrogen peroxide gas plasma sterilization - gives healthcare providers the flexibility to choose any major method for sterilizing trays, electronic medical devices and other applications.
The data revealed that Ultem HU1004 resin has significant performance and aesthetic advantages - in particular, ductility and color stability - over competitive materials such as polyphenylsulfone (PPSU) that are used for STERRAD NX sterilization. Ultem HU1004 resin's validation underscores SABIC Innovative Plastics' commitment to providing its healthcare customers with the highest quality materials to meet their needs for process simplification and performance.
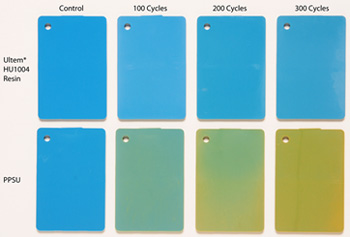 This photo shows the influence on color from increasing exposure to STERRAD® NX®, from baseline, 100 cycles, 200 cycles and 300 cycles. The Ultem HU1004 resin samples on the top row show almost no color change, whereas PPSU displays a shift to yellow as early as 100 cycles.
This photo shows the influence on color from increasing exposure to STERRAD® NX®, from baseline, 100 cycles, 200 cycles and 300 cycles. The Ultem HU1004 resin samples on the top row show almost no color change, whereas PPSU displays a shift to yellow as early as 100 cycles.
"SABIC Innovative Plastics healthcare materials undergo rigorous performance testing in the development of new, high-end applications," said Tom O'Brien, marketing director, Healthcare, SABIC Innovative Plastics. "And based on independent lab testing, Ultem HU1004 resin meets critical industry sterilization demands, including higher-temperature autoclaving, ethylene oxide and gamma radiation for enhanced safety, and low-temperature sterilization processes to protect delicate electronic devices. Ultem HU1004 resin is fast becoming a leading choice among global OEMs for an expanding range of applications because it delivers stable performance and excellent aesthetics regardless of the sterilization method used."
Ultem resins have a long and distinguished legacy in the healthcare industry, where they have demonstrated strength, stability, chemical resistance and dependable performance in applications such as surgical devices (tissue stabilizers, skin staplers and laparoscopic device handles), medical equipment including anesthesia machines and orthopedic devices.
Lab Results Show Superior Mechanicals, Aesthetics for Ultem HU1004 Resin
To validate the mechanical properties and degree of color shift for Ultem HU1004 resin after exposure to the STERRAD NX method, SABIC Innovative Plastics commissioned SPS Medical, an independent testing lab located in Rush, New York. SPS exposed samples of Ultem resin and PPSU to STERRAD NX sterilization in increments of 50 cycles up to 300; these samples were then evaluated by SABIC Innovative Plastics' technical experts.
The findings demonstrate that Ultem HU1004 resin provides superior color stability vs. PPSU. In fact, PPSU showed a color shift up to 10 times greater than Ultem HU1004 resin, becoming significantly yellowed after only 100 STERRAD NX cycles. Ultem resin also delivered superior mechanical properties, including retention of mass and impact performance, compared to PPSU.
Following are key findings from the testing:
Weight Loss (after 150 and 300 STERRAD NX cycles)
- Ultem HU1004 resin lost less than a total of 0.5 percent of its weight after 150 and 300 STERRAD NX cycles
- PPSU had a 2.25 percent weight loss after 150 STERRAD NX cycles and a 7.7 percent loss after 300 cycles.
Tensile Properties Retention (after 150 and 300 STERRAD NX cycles)
- Ultem HU1004 resin retained >99 percent of its tensile strength at 150 cycles and 98 percent at 300 cycles. For tensile elongation at break, the Ultem resin retained 94 percent of performance at 150 cycles and 77 percent at 300 cycles.
- PPSU retained 96 percent of tensile strength at 150 cycles and 91 percent at 300 cycles. The material became brittle and retained only 12 percent of its elongation at 150 cycles, and 11 percent at 300 cycles.
SABIC Innovative Plastics' tough Ultem HU1004 resin is now an excellent candidate material for healthcare applications requiring high temperature autoclaving, ethylene oxide, gamma radiation and low-temperature hydrogen peroxide gas plasma sterilization processes. Testing, conducted by SABIC Innovative Plastics on autoclave sterilization, showed that even after 2,500 autoclave cycles at 134C, Ultem HU1004 resin retained ductility, stiffness and strength.
Ultem HU1004 resin is part of SABIC Innovative Plastics' extensive and growing portfolio of over 50 healthcare resin grades, which are supported by a comprehensive healthcare product policy.