Scientists from the University of Bayreuth have created an oxide glass with extraordinary toughness in collaboration with partners in China and the United States. The investigators succeeded in paracrystallizing an aluminosilicate glass under high pressures and temperatures: the resulting crystal-like structures allow the glass to withstand very high stresses and remain stable under ambient conditions.
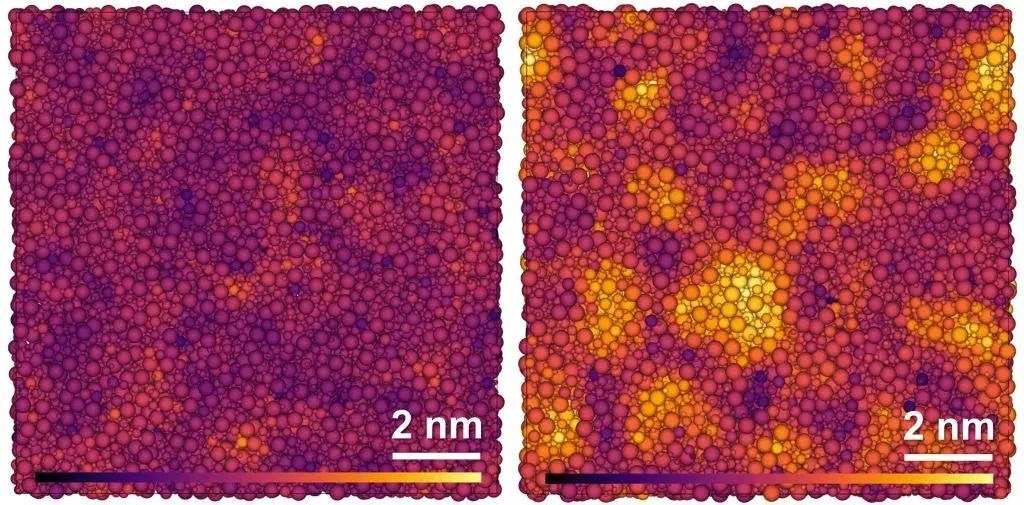 Simulated structure of glassy (left) and paracrystalline (right) grossular. The atoms of the elements oxygen, silicon, aluminum, and calcium (from small to large) are colored lighter the higher the degree of order in the surrounding structure. Image Credit: ©Hu Tang.
Simulated structure of glassy (left) and paracrystalline (right) grossular. The atoms of the elements oxygen, silicon, aluminum, and calcium (from small to large) are colored lighter the higher the degree of order in the surrounding structure. Image Credit: ©Hu Tang.
The results suggested that paracrystallization appears to be a promising process for producing exceptionally break-resistant glasses. The researchers present their findings in Nature Materials, where the German Electron Synchrotron (DESY) in Hamburg also participated.
Glass is an appealing material for modern technologies in many ways. However, because of its brittleness, which invariably leads to cracks and fractures, its potential applications are limited. Attempts to greatly enhance the toughness of glass while retaining its beneficial properties have largely failed to achieve the desired outcome.
The novel approach presented in Nature Materials begins with oxide glasses, which have a somewhat disordered internal structure and are the most commonly used glass materials in the industry. The research group from Germany and China succeeded in giving aluminosilicate, which contains silicon, aluminum, boron, and oxygen, a new structure. They used high-pressure and high-temperature technologies at the University of Bayreuth's Bavarian Research Institute of Experimental Geochemistry and Geophysics (BGI).
The silicon, aluminum, boron, and oxygen atoms grouped to form crystal-like structures at pressures between 10 and 15 gigapascals and temperatures around 1,000 ºC. These structures are referred to as “paracrystalline” since they vary markedly from a completely irregular structure but do not resemble crystals’ clear regular structure.
This intermediate state between crystal structures and amorphous irregularity was cdemonstrated by empirical analyses using spectroscopic techniques as well as theoretical calculations.
The paracrystalline structures in aluminosilicate glass remain even after pressure and temperature are reduced to normal ambient levels. Because of the penetration of these structures into the glass, the toughness of the glass is many times greater than before paracrystallization. It can now reach values of up to 1,99 ± 0,06 MPa (m)¹/². This is a previously unmeasured toughness for oxide glasses. At the same time, the paracrystalline structures have little effect on the transparency of the glass.
The exceptional strength of the glass is explained by the fact that forces acting on the glass from the outside, which would usually cause breakage or internal cracks, are now directed primarily against the paracrystalline structures.
They dissolve sections of these structures and return them to an amorphous, random state. As a result, the glass as a whole gains greater internal plasticity, making it less likely to break or crack when subjected to these or even stronger forces.
Our discovery highlights an effective strategy for developing highly damage-tolerant glass materials, which we plan to pursue with our research in the coming years.
Dr. Hu Tang, Study First Author, University of Bayreuth
“The increase in toughness due to paracrystallization shows that structural changes at the atomic level can have a significant impact on the properties of oxide glasses. At this level, there is great potential for optimizing glass as a material that is far from being exhausted,” concludes Prof. Dr. Tomoo Katsura of the Bavarian Research Institute of Experimental Geochemistry and Geophysics.
Journal Reference:
Tang, H., et al. (2023). Toughening oxide glasses through paracrystallization. Nature Materials. doi.org/10.1038/s41563-023-01625-x.