Gelatin is a flavorless foodstuff derived from collagen acquired from various animal by-products. It is generally used in cosmetics manufacturing, food, photography, and pharmaceuticals. Gelatin is required to meet stringent quality standards in these highly regulated industries. The United States, Japanese, and European pharmacopeias all have monographs that require testing for heavy metals such as Zn, Fe and Cr. In the food industry, the Food Chemical Codex and the European Regulation No. 853/2004 mandate the residue limits for a number of elements, including Cr, Fe, Cu and Zn.

This article demonstrates the capabilities of the Epsilon 1 energy dispersive X-ray fluorescence spectrometer to meet these residue limits.
Instrumentation
Measurements were carried out using the Epsilon 1 energy dispersive X-ray fluorescence spectrometer. This instrument is equipped with a high-resolution silicon drift detector, 50 kV silver anode X-ray tube and 6 filters. Using the Epsilon 1 software, the data was automatically processed.
Standards and Sample Preparation
Eight in-house standards were used to set up calibrations for Fe, Zn, Cr, and Cu in porcine and bovine gelatin. Next, 5 grams of loose powder was weighed into a disposable liquid cup for measurement.
Measurement Conditions
A single measurement condition was employed to analyze Cr, Cu, Zn and Fe in the standards shown in Table 1. The total measurement time per standard was only 5 minutes. The XRF spectrum of one of the porcine standards is shown in Figure 1.
Table 1. Measurement conditions
| Element |
kV |
µA |
Measurement times (s) |
Medium |
Filter |
| Cr, Fe, Cu, Zn |
20 |
250 |
300 |
Air |
thick Al |
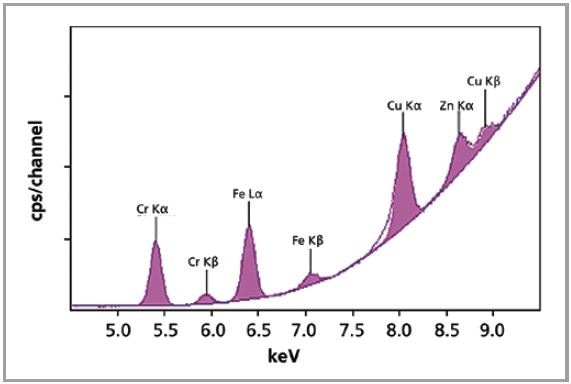
Figure 1. XRF spectrum of porcine gelatin standard.
Calibration Results
The calibration graphs for Cr, Fe, Cu and Zn in gelatin are shown in Figures 2 to 5, respectively. The graphs exhibit very good correlations between the concentrations and the measured intensities. Table 2 shows the detailed calibration results. The root mean square (RMS) value equals 1 sigma standard deviation. The lower limits of detection (LLD; 3 sigma) are also displayed in Table 2.
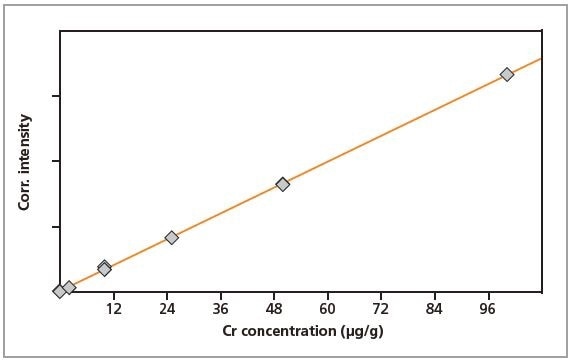
Figure 2. Calibration graph for Cr in gelatin.
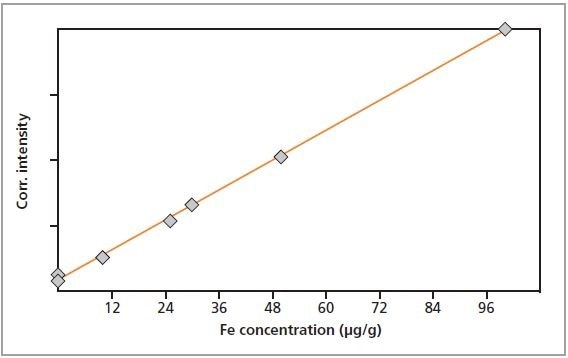
Figure 3. Calibration graph for Fe in gelatin.
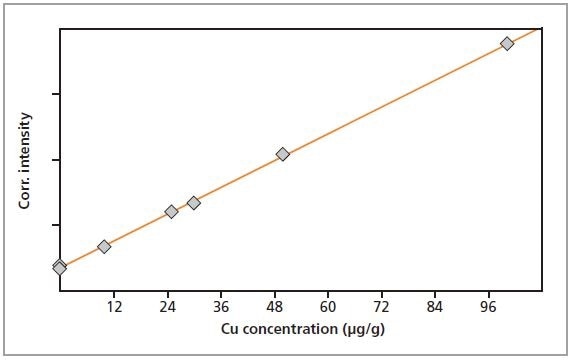
Figure 4. Calibration graph for Cu in gelatin.
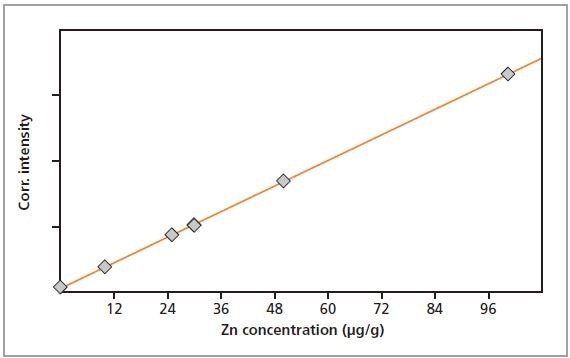
Figure 5. Calibration graph for Zn in gelatin.
Table 2. Calibration details
| Element |
Concentration range (µg/g) |
RMS* (µg/g) |
Correlation |
LLD (µg/g, 300s) |
| Cr |
0.0 - 100 |
0.6 |
0.9999 |
0.4 |
| Fe |
0.0 - 100 |
1.2 |
0.9995 |
0.3 |
| Cu |
0.0 - 100 |
0.9 |
0.9997 |
0.3 |
| Zn |
0.0 - 100 |
0.4 |
0.9999 |
0.4 |
(* RMS: The more accurate calibration have the smaller RMS values.)
Precision
To test the precision of the instrument, measurements were made for a fish gelatin sample for more than 1500 times consecutively over a period of 17 days. Table 3 shows the average concentration and RMS values. All four elements exhibit excellent repeatability. Figure 6 graphically illustrates the stability test for Cr in a fish gelatin sample.
Table 3. Repeatability results of a fish gelatin sample measured over 17 days.
| Elements |
Average concentration µg/g) |
RMS (µg/g) |
Relative RMS (%) |
| Cr |
11.0 |
0.2 |
1.8 |
| Fe |
30.2 |
0.3 |
1.0 |
| Cu |
33.2 |
0.3 |
0.9 |
| Zn |
32.6 |
0.3 |
0.9 |
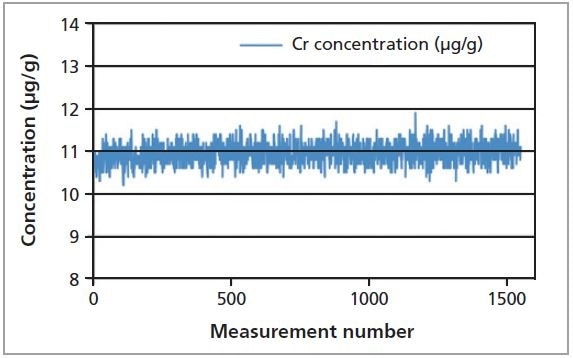
Figure 6. Graphical representation of the repeatability test of Cr in fish gelatin sample measured over 17 days.
Conclusions
It is clearly evident from the above results that the Epsilon 1 energy dispersive X-ray fluorescence spectrometer is highly capable of analyzing Cr, Cu, Zn and Fe in porcine, bovine and fish gelatin.
The sensitivity and high resolution of the silicon drift detector together with powerful software algorithms help quantify traces of metals within the limits stipulated by food and pharmaceutical regulations. Also, the repeatability of the measurements demonstrates the excellent stability of the Epsilon 1 instrument for trace analysis of gelatin.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information on this source, please visit Malvern Panalytical.