Sponsored by PerkinElmerReviewed by Olivia FrostSep 8 2023
A recent article explained the role of Gas Chromatography-Mass Spectrometry (GC/MS) in the analytics laboratory. The current article focuses on reviewing the technology used for preparing volatile samples.

Image Credit: PerkinElmer
Scientists utilizing Gas Chromatography (GC) examine samples' volatile and semi-volatile components. Analyzing specific samples can pose challenges, particularly when the sample matrix is unsuitable for direct injection into the GC. In such cases, a preparatory step is necessary to extract the target analytes from the matrix.
This separation process becomes more straightforward if the target compounds are volatile and have higher vapor pressure than the non-volatile sample matrix. Scientists can leverage the volatility of the target compounds and exploit temperature to separate these analytes from the matrix.
An instrument was developed to heat samples, facilitating this separation process. It also injects these target compounds into the GC column for analysis after the extraction.
Headspace Sampling Technology Definition
Headspace technology emerged in the 1980s, resembling a camera capturing the vapor phase around a specific material or object.
The headspace-gas chromatography (HS-GC) technique was originally invented by Professor G. Machata at the University of Vienna in Austria. The instrumentation of this method was developed by an affiliate of Perkin-Elmer based in Germany.
To illustrate the application of headspace in extracting volatile materials from denser sample matrices, a suitable example is perfume.
Perfume compositions can be intricate, containing water, alcohol, essential oils, and other additives. Injecting such a sample directly into a standard GC injector and column results in the chromatogram depicted in Figure 1.
Generating this chromatogram releases unnecessary compounds—a process viewed as wasteful in terms of time and resources. Moreover, many of these compounds might not be well-suited for gas chromatography, potentially contaminating the system or reacting with the stationary phase in the column.
If a sample of this perfume is placed in a sealed vial and heated moderately for some time, the more volatile compounds will likely move into the gas phase above the perfume sample, also known as the headspace. The compounds with higher volatility will be more concentrated in the headspace.
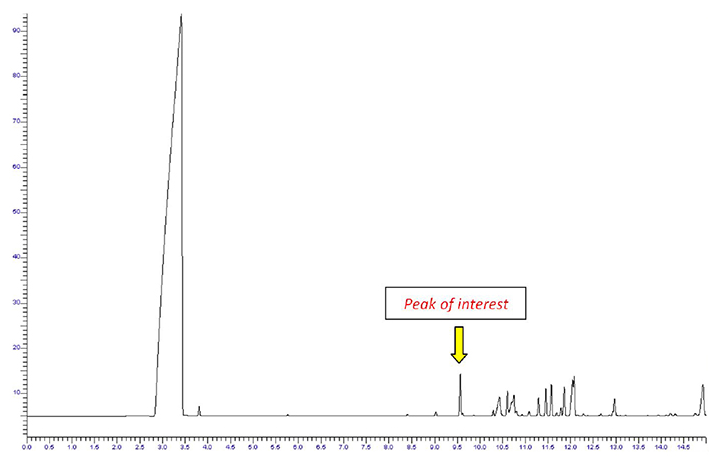
Figure 1. Chromatogram from the direct injection of a perfume sample. Image Credit: PerkinElmer
On the other hand, the components that are less volatile (and more challenging for gas chromatography) and make up the majority of the sample will remain in the liquid phase.
This results in fundamental separation. By extracting a portion of the headspace vapor and injecting it into a gas chromatograph, the less-volatile material entering the GC column is significantly reduced, making the chromatography process smoother, simpler, and faster.
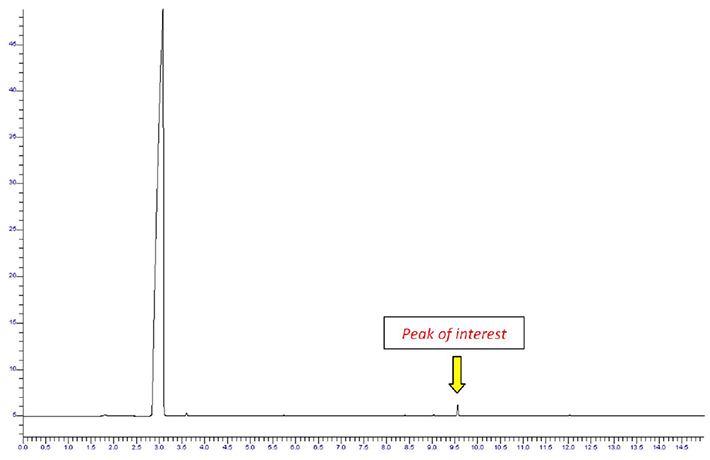
Figure 2. Movement of perfume molecules within a sealed and heated vial. Image Credit: PerkinElmer
To automate this process, a headspace sampling system is employed. It involves heating the sample for a specific duration and extracting a small amount of the headspace vapor from the vial, which is then transferred to the GC column.
The chromatogram in Figure 3 is generated from a headspace sample taken from the same perfume sample that produced the chromatogram in Figure 1.

Figure 3. Chromatography of a perfume sample with headspace sampling. Image Credit: PerkinElmer
Headspace sampling is used to qualitatively and quantitatively analyze volatile substances in samples that can effectively transition into the gas phase in the headspace, whether from a liquid or solid matrix.
This method is also beneficial for analyzing samples in which the complete sample should not be injected into the GC instrument (e.g. samples containing particles).
Headspace sampling is particularly useful for detecting trace amounts of analytes since they naturally become more concentrated during extraction from the semi-volatile matrix.
Common examples of headspace analyses encompass Volatile Organic Compounds (VOCs) from wastewater and contaminated land samples, residual solvents in packaging, pharmaceuticals, and blood alcohol, as well as toxicology screening.
Different Types of Headspace Sampling
There are several headspace sampling methods, with the simplest being Syringe Injection. After heating, this approach uses a gas-tight syringe to withdraw a small vapor volume from a vial's headspace. The vapor is then injected into a GC split inlet.
This can be carried out manually or facilitated by a headspace instrument, which employs a gas syringe to transfer headspace vapor from a thermally equilibrated vial to a GC injector.
Another commonly employed headspace sampling technique found in many headspace instruments is valve and loop injection. These systems employ a needle probe to pierce the vial and pressurize it with gas. Once the vial attains pressure equilibrium, its gases vent into the sample loop, filling it with the sample.
Subsequently, the valve switches, allowing the sample to be carried into the GC inlet by the carrier gas. This method varies from syringe injection as the sample is transported to the GC injector via a controlled flow of carrier gas. Although the sample loop's capacity is constant, it does not accurately reflect the actual injected sample amount.
Pressure and temperature changes, as well as applied splits, directly influence the headspace vapor volume directed into the GC column. Among the three types of headspace (HS) systems, this one proves the most challenging in predicting the injected vapor amount into the GC.
Pressure-balanced headspace sampling technology offers a more straightforward approach to determining injected headspace vapor quantity.
This technology presents a significant advantage in being a single-stage injection technique, introducing sample vapor from the headspace into the GC column without the use of a gas syringe or multiport valves.
Instead, carrier gas pressures are precisely regulated to manage the transfer process, minimizing the variability and contamination sources present in other systems.
Due to the absence of a fixed-volume sample loop, users can adjust sample volumes via settings within the headspace instrument method, showcasing pressure-balanced sampling as more versatile than valve and loop methods.
The Pressure Balance Headspace Sampling Process: Step-by-Step
- The samples are heated in an oven, allowing volatile compounds to attain equilibrium between the vapor and liquid phases inside the sample vials. During this thermostatting period, the heated sampling needle is consistently purged with carrier gas to eliminate any contaminants. Since the column or transfer line is fully inserted up to the needle, it maintains maximum inertness and minimal dead volume.
- In the pressurization phase, the heated sampling needle punctures a sample vial. All vials are pressurized uniformly.
- Following pressurization, a solenoid valve interrupts the flow of carrier gas, and the vial serves as a reservoir of carrier gas. During sampling/injection, as pressure decreases, the sample volume is directly transferred from the vial to the column. This approach prevents the sample from being diluted by carrier gas and avoids sample expansion before injection. Due to the one-stage injection process described earlier, headspace instruments utilizing pressure-balanced sampling produce chromatography with remarkably sharp peaks. This results in unparalleled retention time consistency and peak area uniformity, leading to dependable analysis with minimal carryover, reduced adsorption, and decreased dead volumes.
References and Further Reading
- https://go.gale.com/ps/i.do?id=GALE%7CA95681934&sid=googleScholar&v=2.1&it=r&linkaccess=abs&issn=15275949&p=AONE&sw=w&userGroupName=anon%7E234d4d86
- https://blog.perkinelmer.com/posts/chromatography-explained-headspace-sampling-technologies-for-volatile-sample-prep/
- https://www.perkinelmer.com/libraries/APP_ResidualSolventsPharmaUSP467

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer.
For more information on this source, please visit PerkinElmer.