In this interview, industry experts Anthony Buzzerio, Venkat Nandivada, and Rohit Ramnath break down the science behind epoxy selection and precision dispensing, providing an engaging look into the smarter materials and processes improving the reliability of wearable medical sensors.
What are the challenges in manufacturing wearable medical sensors, and how does this case study address them?
Anthony Buzzerio: The long-term durability of wearable medical technology and the miniaturized sensors it relies upon is fundamentally challenged by the need for an improved manufacturing process. This process must be able to protect the delicate internal components from harsh environmental factors such as humidity, dust, and mechanical impact.
This case study examines the selection and precise application of a biocompatible, non-cytotoxic epoxy encapsulant for wearable devices. It highlights the crucial roles of both a specialty adhesive manufacturer (Master Bond) and a fluid dispensing leader (Nordson EFD) in optimizing the automated dispensing process to achieve the accuracy and consistency required for high-quality, scalable production.
What were the requirements for the epoxy for the wearable sensor?
Rohit Ramnath: The epoxy for this wearable sensor had to meet several critical requirements. Low viscosity with capillary flow was essential for the material to fill intricate spaces without voids. It required a cure temperature below 80 °C to protect sensitive electronic components from heat damage.
Additionally, the material needed to be optically clear and, most importantly, non-cytotoxic, to ensure it was safe for skin contact. Finally, the dispensing process required automation to consistently apply a precise amount of material for each sensor.
Which epoxy encapsulant was selected for the application?
Venkat Nandivada: Master Bond’s EP21LSCL-2Med, a two-component epoxy, met the overall application requirements. The product’s ISO 10993-5 non-cytotoxicity rating made it easier to qualify for the wearable sensor. The epoxy’s low-temperature heat curing below 80 °C was crucial for protecting sensitive electronics within the wearable device.
With a low mixed viscosity of 500-1000 cps, it was ideal for capillary action, ensuring it flowed easily into intricate spaces without creating voids. The material was packaged in pre-mixed and frozen syringes, delivered in 3cc Nordson EFD syringes, making the process highly efficient and reducing waste.

The 6-8 hour working life of Master Bond EP21LSCL-2Med (stored at -40 °C) was a key factor in selecting the dispense solution. Once thawed, the material could not be refrozen. Image Credit: Master Bond Inc.
What factors influenced the choice of dispensing technology?
Anthony Buzzerio: The dispensing technology needed to ensure that the material could flow easily around sensor components and fill intricate spaces without leaving voids. This required a comprehensive solution including a fluid dispenser, valve, automation, and tip technologies.
The six- to eight-hour working life of Master Bond EP21LSCL-2Med was a key factor in selecting the dispense solution. The material, once thawed from its -40 °C freezer storage, could not be refrozen. This influenced the choice of a system that could manage the material within its limited working life.
Why was the Nordson Ultimus V chosen for this specific application?
Anthony Buzzerio: The Ultimus V was selected to optimize the use of Master Bond EP21LSCL-2Med within its working life. This high-precision benchtop fluid dispenser provides electronic control over dispensing time, air pressure, and vacuum, ensuring consistent application even as the fluid's viscosity changes. Once programmed, its full electronic press regulation automatically adjusts the air pressure as the epoxy thickens, maintaining accuracy from start to finish.
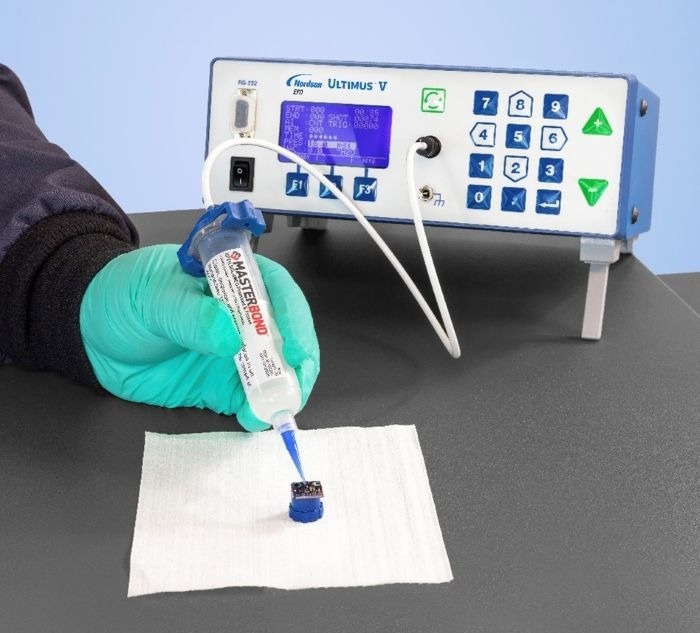
The Ultimus V fluid dispensing solution was recommended because the shot size stays consistent regardless of changes in viscosity in the EP21LSCL-2Med. Image Credit: Nordson EFD
How did Master Bond address the challenge of air bubbles in its pre-mixed and frozen epoxy?
Venkat Nandivada: To minimize air bubbles, Master Bond implemented rigorous degassing protocols, including centrifugation at 2,000–3,000 rpm for 1-2 minutes, which effectively removed entrapped air from the adhesive. The degassed mixture was immediately frozen and packaged in 3cc Nordson EFD syringes, ensuring a uniform, bubble-free material for automated dispensing.
This method benefited the end user by eliminating on-site mixing, which is prone to variability and contamination. This improvement in production uptime, consistency, and precision ensured the encapsulant performed flawlessly in a demanding medical application.

A careful thawing process and use of a centrifuge eliminated the possibility of air bubbles introduced by FTVs (Freeze Thaw Voids). Image Credit: Nordson EFD
What steps were taken to prevent air bubbles from forming during the dispensing process after the epoxy was thawed?
Anthony Buzzerio: To avoid air bubbles, also known as freeze-thaw voids (FTVs), Nordson advised the customer to carefully thaw the syringes and not to rush the process. Additionally, to ensure no air pockets, EFD recommends centrifuging the material for one to two minutes before use with the ProcessMate 5000 Universal Centrifuge.
Can you please share with us the results of this collaborative case study?
Rohit Ramnath: The collaborative application of Master Bond’s innovative pre-mixed and frozen epoxy alongside Nordson’s best-in-class dispensing technology proved instrumental in the customer's success in manufacturing their wearable sensor solution. This synergy effectively addressed the challenge of air bubble elimination, which ensured that the encapsulation process was state-of-the-art. The sensors were fully secured and offered the highly protective capabilities required in the application.
Have a dispensing question you'd like us to answer? Don’t hesitate to submit your question or comment at [email protected].
Have an adhesive question you'd like us to answer? Don’t hesitate to submit your question or comment at [email protected].
About Anthony Buzzerio 
Anthony Buzzerio is an Application/Systems Engineer II at Nordson EFD. He has 4.5 years in the fluid dispensing industry. Buzzerio holds a Bachelor of Science in Engineering from Roger Williams University, Bristol, RI.
About Rohit Ramnath 
Rohit Ramnath is a Senior Product Engineer at Master Bond with over 13 years of experience. He manages new product development and troubleshoots customers’ applications in the medical, aerospace, electronics, and optical industries. He received a master’s degree in chemical engineering from Carnegie Mellon University.
About Venkat Nandivada 
Venkat Nandivada is the Manager of Technical Support at Master Bond and has over 15 years of experience in analyzing application-oriented issues and providing product solutions for aerospace, electronics, medical, optical, and other companies. He earned a master’s degree in chemical engineering from Carnegie Mellon University.

This information has been sourced, reviewed, and adapted from materials provided by Master Bond Inc.
For more information on this source, please visit Master Bond Inc.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.