The AutoChem III is a single piece of analytical equipment that acts as a catalyst characterization laboratory.
- Improved sample and gas temperature control, innovative gas mixing, and 100 % improvement in sensor sensitivity result in industry-leading accuracy
- Save time with quick cooling, a non-cryogenic moisture trap for TPR, and more available gas inlet ports to readily switch between instruments
- Increase user safety by operating without cryogenic liquids, glass vacuum dewars, and labor-intensive hot fitting operations to increase operator safety
Description
AutoChem III
The AutoChem from Micromeritics is the most automated and accurate system for temperature-programmed reactions and chemisorption. It is thus the most widely used and highly cited system for catalyst reactivity characterization.
With a design that will save users hours of daily labor, produce the most reproducible and sensitive measurement possible, and improve operator safety, the all-new AutoChem III meets and exceeds that performance.
- Pulse Chemisorption
- TPR
- TPO
- TPD
- TPSR
- Dynamic B.E.T.
- Breakthrough Curve
- Metal Dispersion
- Metal Surface Area
- Active Surface Area
- Crystallite Size
- Heat Of Desorption
- Activation Energy
- B.E.T. Surface Area
- Metal-Supported Catalysts
- Acid or Base Catalyzed Reactions
- Oxide or Zeolite Catalysts
- Advanced Battery Anode Materials
- Fuel Cell Catalysts
Benefits
Quick and Easy
Users can save hours a day by using the new AutoChem III, which is designed to simplify and expedite critical operations, allowing users to spend more time making progress and less time taking measurements.

Rapid Turnaround Time With Autocool. Image Credit: Micromeritics Instrument Corporation
The NEW AutoCool, an integrated gas-fed system, quickly cools sample tubes before and during tests. AutoCool is usually 30 minutes quicker than other systems and does not require any liquids or external assistance.

Never Prepare Another Vapor Capture Slush Bath. Image Credit: Micromeritics Instrument Corporation
The NEW AutoTrap catches vapor efficiently and does not require manual slush bath preparation. Conventional vapor capture techniques require labor-intensive slush-bath preparation—that is, manual mixing of isopropanol and liquid nitrogen. The AutoTrap's zeolite bed can be regenerated in situ, can effectively capture vapors, and can be used for multiple experiments without interruption.

Program What You Envision, Visualize What You Have Programmed. Image Credit: Micromeritics Instrument Corporation
To ensure that the method aligns with the vision, the new MicroActive method editor includes an easy-to-use process depiction that displays the instrument’s programmed state at each stage of the method.
Making Accuracy Easy:
Exclusive Automated Detector Calibration
The automatic detector calibration of the AutoChem III simplifies quantitative accuracy. Conventional systems need to be calibrated using numerous runs of reference materials or one-point offsets that fail to account for variations in pressure or temperature.
The AutoChem III produces accurate results via an entirely automated process using the patented gas blending capabilities of the system. This includes compensation for injection loop pressure and temperature to guarantee the highest calibration and accuracy. The procedure yields more precise findings compared to other designs. It is also quick and automated, requiring no involvement from the operator.

Loading Samples Is a Snap. Image Credit: Micromeritics Instrument Corporation
Compared to conventional designs, which have half as many individual parts and no threaded fittings, the patented new KwikConnect facilitates sample tube assembly more quickly, easily, and reliably. Installation and removal are quicker and easier, minimize the risk of sample tubes breaking, and offer peace of mind that the snap lock closure has sealed the system completely.

Ready to Run With 18 Available Gas Streams. Image Credit: Micromeritics Instrument Corporation
Do not waste time disconnecting and switching gas lines; instead, have what is needed on hand when it is needed. The AutoChem III has 18 accessible gas streams, so users are always prepared to perform the next reaction.
Having the properly blended gas on hand also means users will not introduce mistakes from poorly designed external gas connections, and they will not jeopardize data accuracy by blending gases, which introduces errors from mass flow controllers unnecessarily.
Better Measurements
Better Measurements for More Confident Decisions
The AutoChem III generates findings that drive confident decisions. The greatest available measurement precision and repeatability–achieved under conditions that mirror the reaction environment–provide users with the certainty to respond confidently.
Precision Temperature Control
Exacting thermal accuracy is required to recreate reaction conditions while keeping valuable catalyst materials from deactivating. The AutoChem III outperforms all other systems in every critical performance metric.
- Superior control accuracy without overshoots
- Four independently controlled gas stream temperature zones eliminate vapor condensation and enhance measurement stability
- Widest range of temperatures: -100 °C to 1200 °C
- Widest range of heating rates: 0.1 °C/min to 100 °C/min
- Repeatable temperature profiles
- Accurate determination of activation energy, Ea
- Local sample temperature measurement
Most Accurate Gas Stream Composition
To prevent carryover and signal tailing when changing gas flow conditions, the AutoChem III has a very low gas flow route volume. This ensures accurate gas stream composition, even when altering setups between experiments.
With 18 accessible gas inlets, users will have the gas composition they need ready, without introducing inaccuracies associated with in situ gas blending.
Better Temperature Control at Every Step
- Furnace: to simulate reaction conditions
- Vapor: to control vapor composition
- Gas Stream: to maximize detection sensitivity
- Detector: to ensure robustness
See More of the Reaction with The Most Sensitive Chemisorption System in the World
The AutoChem III includes a new thermal conductivity detector (TCD) that is 110 % more sensitive than earlier models. This allows for the utilization of smaller sample masses, precise secondary reaction detection, and improved accuracy of catalyst characteristics such as site coverage.
A reference stream with a specialized mass flow controller (MFC) that offers a steady reference to the sample stream improves detector sensitivity. Other systems use a shared carrier stream for both the reference and the signal channels, causing interference between the measurement and reference streams, which in turn leads to signal instability.
The temperature-controlled TCD is a durable sensor that has an extended lifespan and built-in defense against operational mistakes, such as gas flow leaks, which lead to the early failure of 4-element detectors utilized in subpar designs.

Image Credit: Micromeritics Instrument Corporation
Continuous Controlled Vapor Dosing
With the available vapor generator featuring automated vapor calibration, injection repeatability greater than 1 %, and all-new continuous dosing capabilities, users can achieve quicker analysis and more comprehensive characterization of surface selectivity and functionality.

Image Credit: Micromeritics Instrument Corporation
This system produces uniform streams of saturated vapors such as water, amines, alcohols, or organics that are utilized to prepare samples for TPD or as the reaction gas stream.
The new continuous dosing feature allows for quicker and more uniform vapor dosage compared to older systems that are restricted to discrete vapor stream pulses.
Move Quickly From Data to Decision
Utilize the exclusive AutoChem data analysis software from Micromeritics to quickly go from experimental data to material properties. Get all the information required using:

Image Credit: Micromeritics Instrument Corporation
- Interactive peak analysis including deconvolution, integration, limit selection, and baseline definition
- Integrated analytical models for total pore volume, pulse chemisorption, heat of desorption, activation energy, BET, percentage dispersion, metal surface area, crystallite size, Langmuir, first-order kinetics, and more
- Smooth incorporation of mass spectrometry data
- Detailed, configurable graphical reports
Improving Operator Safety
At every level of the measuring process, the AutoChem III increases operator safety by minimizing opportunities for exposure and potentially dangerous situations.
No Cryogenic Liquids
The new AutoTrap eliminates moisture without cryogenic liquids like liquid nitrogen. Additionally, the AutoTrap removes the requirement for slush bath preparation, which necessitates vigorous mixing of solvents and alcohols in glass vacuum flasks.
Cool to the Touch Third-Party Tested and Verified
After each experiment, the new AutoCool rapidly returns sample tubes to ambient temperature so that users can quickly swap out samples and begin the next experiment without having to handle hot glass sample tubes.
The KwikConnect sample tube retention system also eliminates fumbling with individual adapter parts and threaded connections, allowing users to release the tube in a single move.
Third-Party Tested and Verified
Products from Micromeritics are third-party tested to ensure that they meet the strictest operational safety and regulatory requirements. Install and operate the system with the assurance that it will satisfy and exceed electrical safety and compatibility requirements without the need for further certifications or evaluations.

Image Credit: Micromeritics Instrument Corporation
Features
AutoChem III Features
- A temperature-controlled corrosion-resistant detector that is compatible with corrosive gasses and protected from gas leaks that would damage alternative designs, offering long operational life and high reliability.
- With two times the sensitivity of other options, the high-sensitivity thermal conductivity detector (TCD) allows users to measure smaller sample quantities, identify secondary reactions, and have more confidence in their findings.
- 18 total gas inlets—six for preparation, carrier, and loop gases—allow for consecutive tests of various kinds and reduce the time between experiments.
- Exclusive AutoTrap offers improved moisture removal for TPR investigations with a system that is simple to operate and saves hours every day.
- The dynamic clamshell furnace offers temperature control up to 1200°C and adjustable heating rates from 0.1 °C/min to 100 °C/min with the lowest temperature overshoot available.
- Integrated auto-cooling cools the furnace and sample more quickly than forced air alone without the utilization of cryogenic liquids, saving approximately thirty minutes for each experiment.
- Internal gas temperature control in four distinct zones reduces condensation during investigations with vapor and enhances overall signal stability.
- When modifying the composition of the gas stream, the lowest internal gas volume reduces tailing and offers the maximum peak resolution.
- Sample tube attachment is quick, simple, and safe with the KwikConnect retention system, as it has fewer parts than standard designs and does not require threaded connections.

Image Credit: Micromeritics Instrument Corporation
Specifications
AutoChem III Specifications
Source: Micromeritics Instrument Corporation
| . |
. |
| Temperature |
Ambient to 1200 °C |
| Temperature Ramp Rates |
-100 °C to 800 °C: up to 100 °C/min
800 °C to 1000 °C: up to 50 °C/min
1000 °C to 1200 °C: up to 25 °C/min |
| Preparation gases |
6 inlets: H2, O2, He, Ar, H2/Ar, and more |
| Carrier gases |
6 inlets: He, Ar, H2/Ar, and more |
| Analysis (loop) gases |
He, H2, CO, O2, N2O, NH3/He, and more |
AutoChem III Capabilities
- Reduction, Oxidation Temperatures
- Acid site strength distribution: Lewis/Brønsted acid site distribution
- Breakthrough Curve Measurement
- Activation Energy
- Pulse Chemisorption
- Temperature-programmed reactions: TPR, TPO, TPD, TPSR
- Strong Chemisorption: Reactive metal area, dispersion, crystallite size
- Active site surface concentration
Optional Capabilities
- Enhanced Chemical Resistance
- B.E.T. Surface Area
- CryoCooler -100 °C to 1200 °C
- Detection by Mass Spectrometry
- Continuous or Pulsed Vapor Dosing: water, alcohol, amines, aromatic organics, and more
Configurations
Mass Spectrometer
A direct method of determining the identification and concentration of certain reaction products is mass spectrometry. This is particularly helpful when studying an unknown reaction or a reaction that produces several products.
The single quadrupole mass spectrometer with a heated transfer line offers the detection of mass fragments up to 200 amu and data collection that is incorporated with the operation of the AutoChem III.
A generic mass spectrometer communication port is also included in the AutoChem III to facilitate coordination with an existing mass spectrometer in a laboratory.
Cryocooler
Begin tests with controlled liquid-nitrogen cooling at temperatures as low as -100 °C.
Vapor Generator
Prepare samples for analysis or conduct measurements in the presence of pulsed or continuous vapor streams such as alcohol, pyridine, water, aromatic organics, and other substances.
Enhanced Corrosion Resistance (ECR)
An advanced version of the AutoChem III is offered with improved corrosion resistance for reaction chemistries that call for extremely aggressive gas compositions. To offer the best stability under the hardest working conditions, wetted materials are made of very stable perfluoroelastomers, highly resistant Hastelloy, and inert-coated stainless steel.
Applications and Methods
Applications
Net Zero Technologies
The advancement of carbon dioxide (CO2) mitigation and the hydrogen economy, which will facilitate a sustainable energy future, depend on the development of efficient and effective catalysts.
The AutoChem III can be used for optimizing H2/O2 adsorption and dissociation on electrolysis electrodes, determining if desorption occurs close to reaction conditions, quantifying acid or base sites to maximize reactivity and selectivity, and more.
Fuel Cells
Pt/C, PtRu/C, and PtRuIr/C are examples of platinum-based catalysts. They are frequently characterized by temperature-programmed reduction to ascertain the number of oxide phases and pulse chemisorption to determine metal dispersion, average crystallite size, and metal surface area.
Partial Oxidation
The following are the characteristics of the manganese, cobalt, bismuth, iron, copper, and silver catalysts used in the gas-phase oxidation of ammonia, methane, ethylene, and propylene: Temperature-controlled desorption and oxidation, oxygen dissociation, and desorption heat.
Catalytic Cracking
Large hydrocarbons are converted to gasoline and diesel fuel by acid catalysts like zeolites. Ammonia chemisorption and temperature-programmed desorption are two methods used to characterize these materials.
Catalytic Reforming
To produce hydrogen, aromatics, and olefins, catalysts containing platinum, rhenium, and tin on silica, alumina, or silica-alumina are utilized.
Isomerization
To convert linear paraffins into branched paraffins, catalysts like small-pore zeolites (mordenite and ZSM-5) containing noble metals (usually platinum) are utilized.
Hydrocracking: Hydrodesulfurization, and Hydrodenitrogenation
Hydrocracking catalysts are used to process feeds containing polycyclic aromatics that are unsuitable for conventional catalytic cracking methods. These catalysts are often made of metal sulfides, such as nickel, tungsten, cobalt, and molybdenum.
Water Gas Shift Reaction
An essential component of the hydrogen lifecycle and the drive toward net-zero technology is the water-gas shift reaction. The combination of catalysts (often iron-chromia and copper-zinc-alumina) is characterized by pulse chemisorption and TPR to maximize activity.
Methods

Temperature Programmed Reactions. Image Credit: Micromeritics Instrument Corporation
By altering the composition of the gas stream, the suite of temperature-programmed reactions is utilized to quantify reactivity as a function of temperature. The consumption of reactive gases, the production of reaction products, and the desorption of bound species all cause changes in the composition of the gas stream as temperature rises.

Temperature-Programmed Desorption Measurement (TPD). Image Credit: Micromeritics Instrument Corporation
It is possible to desorb previously adsorbed species by raising the sample temperature under flowing inert gas. Among the most popular uses is ammonia TPD. To determine the relative acid site strength of materials like zeolites, a sample is first saturated with ammonia during the preparation process and then heated to desorb-bound ammonia.
Similarly, CO2 desorption reveals the strength of basic sites. It is also possible to characterize carbonates to remove CO2 or hydrides for the storage of hydrogen by bulk decomposition into the gas phase.

Temperature Programmed Reduction (TPR). Image Credit: Micromeritics Instrument Corporation
The TPR measurement is a particular instance of a temperature-programmed reaction in which an oxide sample is exposed to a mixture of hydrogen and an inert carrier, often argon. Water vapor is produced when hydrogen is extracted from the gas stream.
The AutoTrap captures the water vapor, and the hydrogen lost from the carrier stream is quantified. The temperature, time, and activation energy needed to prepare a heterogeneous catalyst from its native oxide state into the active zero-valence metal are specifically provided by this measurement.
Temperature Programmed Oxidation (TPO)
When oxygen in the sample gas stream is consumed in a TPO experiment, it typically reacts with various types of carbon to create CO or CO2. To maximize process conditions and assess the reactivity of metal oxide catalysts, TPO experiments are crucial.
Since the temperature of oxidation is correlated with the reactivity of carbon-carbon bonds, it is a useful tool for distinguishing different carbon forms. Amorphous, nanotube, filament, and graphitic carbon may be identified with TPO; this is particularly useful for carbons that develop on catalysts.

Pulse Chemisorption. Image Credit: Micromeritics Instrument Corporation
Via temperature and gas exposure, a sample is prepared in situ to a defined initial state (such as pure oxide or valence metal). The system delivers known-volume reactive gas pulses to the sample and quantifies the volume of gas utilized in each pulse.
B.E.T. Surface Area: Physisorption
The N2 that is added to or removed from a flowing gas stream at ambient or liquid nitrogen temperatures, respectively, is measured using the flowing or dynamic method by AutoChem III to determine the B.E.T surface area.
Since they reflect the basic physical form of the catalyst and support the available contact area for reactivity, basic physical surface area measurements are crucial for the development of catalysts. This is also a key basic measurement for granular and porous materials of all types.
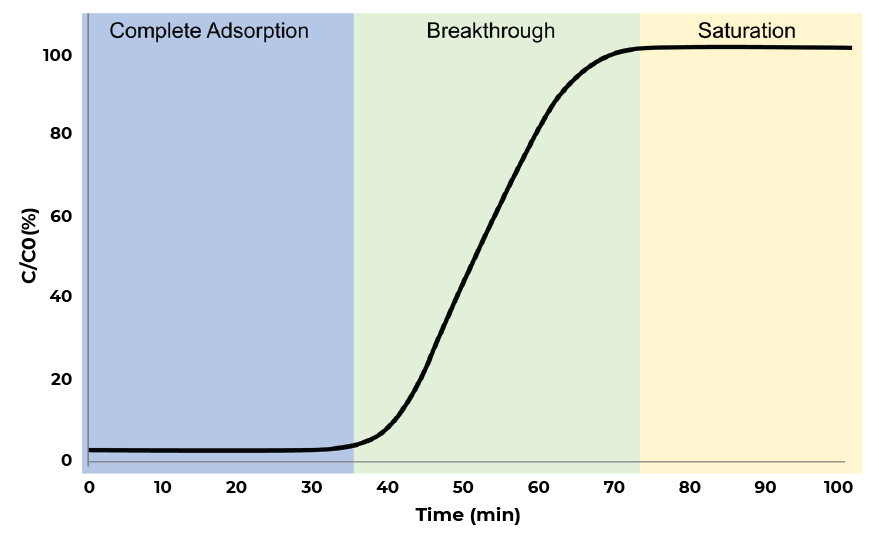
Breakthrough Curve Analysis. Image Credit: Micromeritics Instrument Corporation
A potent method for determining a material’s adsorption capacity under dynamic flow conditions is breakthrough analysis. During an experiment, breakthrough analysis enables users to accurately regulate the temperature, pressure, and gas flow rates. This enables operators to analyze absorbates under process-relevant conditions, providing them with the tools needed to enhance their systems and absorbent materials.
The breakthrough also makes it simple for users to get multicomponent equilibrium adsorption data, which enables them to ascertain the materials’ selectivity and adsorption kinetics.
THE MOST ADVANCED CHEMISORPTION SYSTEM IN THE WORLD| AUTOCHEM III
Video Credit: Micromeritics Instrument Corporation