Lithium-ion batteries (LIBs) stand out among all energy storage technologies thanks to their exceptional volumetric and gravimetric charge capacities, enabling various mobile electronic devices to utilize them.
The electrochemical performance of LIBs is constrained and controlled by concentration gradients that are produced as a result of anionic polarization. An effective method for reducing and preventing the overpotential caused by salt concentration polarization is the immobilization of anions by bonding them to a polymer matrix. However, the reduction in mobile ions and their transportation through the polymer matrix frequently harm the total ionic conductivity of the single lithium-ion polymer electrolytes (SLICPE).
However, the gradients in lithium salt concentration in the electrolyte continue to cause all the issues related to polarization. To serve as liquid electrolytes devoid of solvents, various room-temperature (RT) lithium ionic liquids (LiILs) were created. However, the described LiILs have only been applied as salts or additions to popular multi-component organic solvent electrolytes up to this point. These experiments did, however, demonstrate the notion of lithium liquid ionic liquids; nonetheless, they did not exhibit the requisite electrochemical properties as single-component electrolytes for lithium batteries.
About the Study
In this study, the authors demonstrated the utility of a single-component electrolyte in lithium batteries based on novel lithium borate ionic liquids. The core tetracoordinate boron atom of this type of LiILs was designed with oligoethylene glycol groups, one alkane group, and fluorinated electron-attracting groups. The improved borate, Li+ LiILs, exhibited electrochemical stability higher than 4 V, high lithium transference numbers, tLi+ = 0.4–0.5, and an ionic conductivity greater than 10-4 S cm-1 at 25 °C. In plating/stripping tests, some of the LiILs exhibited stable polarization profiles and great compatibility with lithium-metal electrodes.
The team explored the proposed LiIL as single-component electrolytes in the lithium-metal battery cells with discharge capacities of 124 and 75 mAh g-1 at a C-rate of 0.2 C and 65 °C, respectively, with negligible capacity loss in Li0/LiIL/lithium titanate and Li0/LiIL/lithium-iron phosphate cells. The proof of concept for a new family of LiIL salts that can be used as monocomponent electrolytes in batteries was discussed. The previously described approach for producing lithium borate salts from the straightforward reaction of trialkoxy borates with n-butyl lithium was used to create the LiILs.
The researchers used tetracoordinate borate groups, a variable electron-withdrawing group and its interaction energy with Li+, and an alkane substituent connected by a boron-carbon covalent bond, to create several new lithium ionic liquids with highly delocalized anionic. Organolithium compounds were used to create the alkane substituent, which stabilized the anionic center, reduced its hygroscopicity, and provided Li+ to synthesize borate complexes.
The impact of different substituents on intrinsic electrochemical parameters was studied. It was shown that the proposed LiILs had the required ability for lithium plating-stripping and could be employed as single-component electrolytes for lithium batteries with good cyclability and performance with well-known electrode materials.
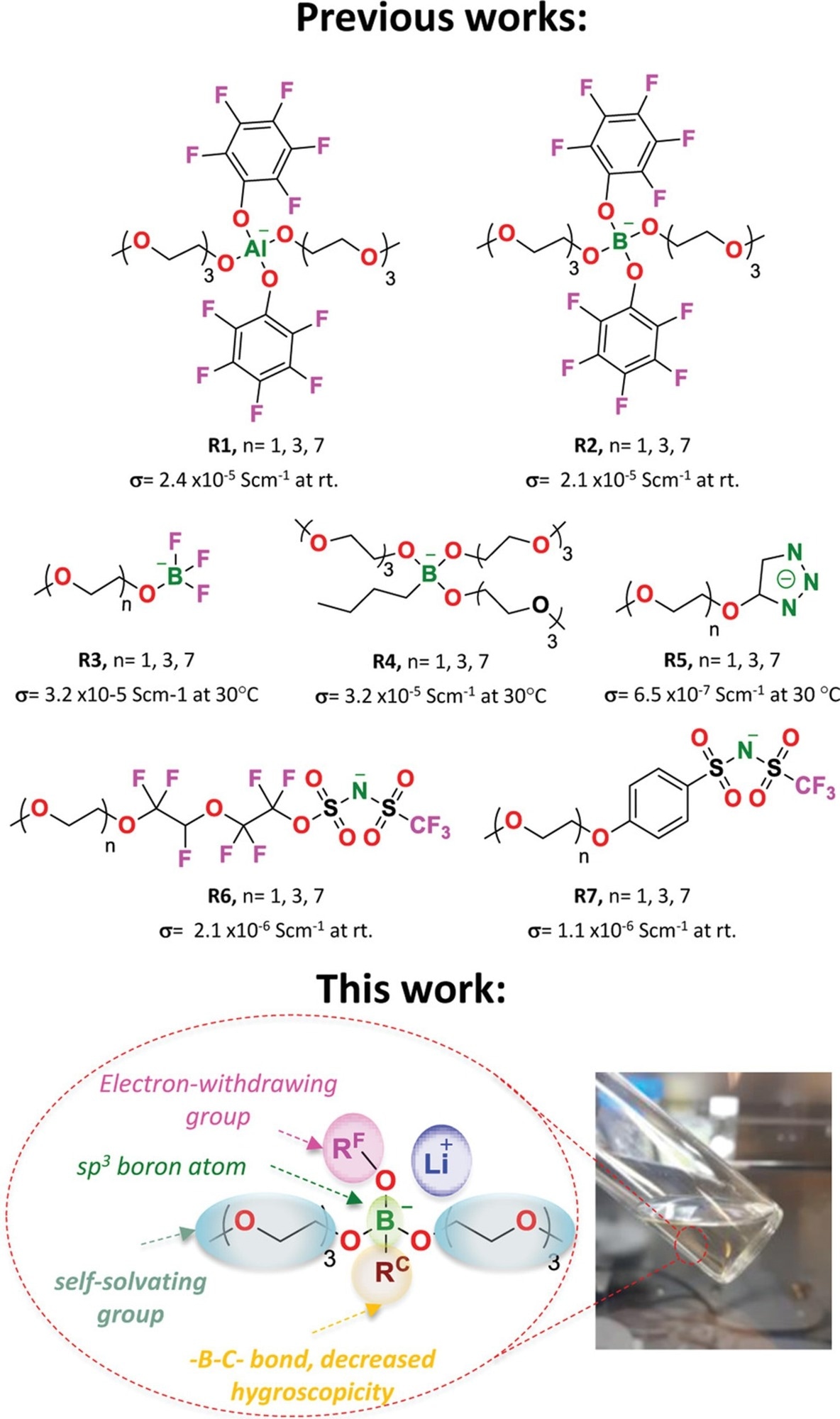
Image Credit: Guzmán-González, G et al., Advanced Energy Materials
Observations
The ability to collect steady charge and discharge values of 172 and 124 mAh g-1 with a loss capacity of 5% after 20 cycles were achieved despite the presence of a significant cell activation overpotential and a poor Coulombic efficiency of 80%. After 30 cycles, the charge capacity of 50 mAh g-1 for the initial cycle decreased by 4% from its original value. After 210 and 910 hours, respectively, the cells built with LiIL2 and LiIL8 electrolytes showed overpotential values higher than ±0.9 V versus Li0/Li+.
Despite the LiIL9 electrolyte's reduced ionic conductivity, it allowed for steady cycling for roughly 200 hours before the cell overpotential steadily increased from ±0.4 to ±1.1 V. The LiIL4 electrolyte demonstrated that the ternary hexafluoro-2-methyl-2-propanoxy as an electron-withdrawing substituent reduced the activation energy and produced greater ionic conductivity values up to 1.04 x 10-4 S cm-1 at 25 °C. With only one equal quantity of CF3 groups as the electron-withdrawing substituent, the LiIL5 produced from the ternary alcohol 2-trifluoromethyl-2-propanol had a poor ionic conductivity value of 2.43 x 10-5 S cm-1.
In plating/stripping studies, the LiILs demonstrated good compatibility with lithium-metal electrodes with consistent polarization profiles due to their high ionic conductivity. Lower ionic conductivity measurements for LiIL3 were 5.31 x 10-5 S cm-1. The new family of borate-Li+ LiILs showed strong ionic conductivity values, lithium transference numbers, and electrochemical stability.
The proposed LiILs underwent successful initial testing as single-component electrolytes in lithium-metal batteries and demonstrated discharge capacities of 124 and 75 mAh g-1 in Li0/LiILs/LTO and Li0/LiILs/LFP cells, respectively, at a C-rate of 0.2 C and 65 °C with a negligible capacity loss.
Conclusion
In conclusion, this study looked into a new family of ionic liquid borate-lithium salts as a single-component electrolyte for lithium batteries. A core tetracoordinate boron atom was asymmetrically substituted with two oligoethylene glycol units, one fluorinated electron-attracting group, and one alkane group in the design of the proposed LiILs.
The authors determined the ideal equilibrium between the fluorinated groups' ability to remove electrons, the ethoxide groups' ability to dissolve substances, and the interfacial compatibility of the stabilizing aliphatic groups.
Reference
Guzmán-González, G., Alvarez-Tirado, M., Olmedo-Martínez, J. L., et al. Lithium Borate Ionic Liquids as Single-Component Electrolytes for Batteries. Advanced Energy Materials (2022). https://onlinelibrary.wiley.com/doi/10.1002/aenm.202202974
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.