Traditional light microscopy has distinct limits due to the diffraction of light, and as such, it cannot resolve features that are less than 200 nm apart.
Microscopy technique development has historically focused on improvements in imaging techniques, facilitating the resolution of individual molecules. The beginning of the 21st century saw the development of super-resolution microscopy, allowing imaging at a resolution beyond the 200 nm limit.7,8
Unfortunately, however, even super-resolution techniques have limitations, including limited imaging depth, a limited field of view, incompatibility with multicolor experiments, high energy illumination intensity requirements, and slow acquisition time.
In order to address these issues, a handful of creative research groups went in a different direction, exploring approaches to straightforward, standardized nanoscale imaging. One result of this work was Expansion Microscopy (ExM) - an imaging protocol that enables conventional light microscopes to resolve densely packed or sub-diffraction limited (<200 nm) details which were previously impossible to distinguish.
Edward Boyden's team at MIT reported the development of this new modality of magnification in 2015. It was developed while the team attempted to overcome challenges in mapping molecules across large scale neural circuits in the brain; and Boyden's research group successfully developed a means of magnifying the specimen itself, rather than magnifying the emitted signal from the specimen.2
Table 1. Glossary.
| Glossary: |
|
| AcX |
acryoyl-X, SE, 6 - (acryoyl amino hexanoïc) ester succinimidylique (a crosslinking agent that reacts with amines of proteins and be copolymerized in polyacrylamide matrices) |
| DiExM |
differential expansion microscopy |
| DMAA |
N,N dimethylacrilamide acid - (nonionic acrylic monomer that can make swellable polymeric particles) |
| ExFish |
expansion microscopy fluorescence in situ hybridization |
| ExM |
expansion microscopy |
| FP |
fluorescent protein |
| GA |
glutharaldehyde (crosslinking fixative, penetrate membran more slowly than PFA) |
| HCR |
hybridization chain reaction |
| iExM |
iterative expansion microscopy |
| IF |
immunofluorophore |
MA
NHS |
methacrylic acid N-hydroxysuccinimide ester (crosslinking agent that reacts with amines of proteins and can be copolymerized in polyacrylamide matrices) |
| MAP |
maximum analysis of the proteome |
MBAA
or BA |
N’N Methylenebis (acrylamide) /(bisacrylamide) crosslinking agent that polymerize with acrylamide and creates crosslinks within the polyacrylamide gel) |
| PFA |
paraformaldehyde (crosslinking fixative, preserves the secondary/tertiary structures of proteins) |
| ProExM |
protein-retention expansion microscopy |
| SDS |
sodium dodecylsulfate (anionic surfactant useful to denature and dissociate proteins) |
| SRRF |
super resolution radial fluctuations |
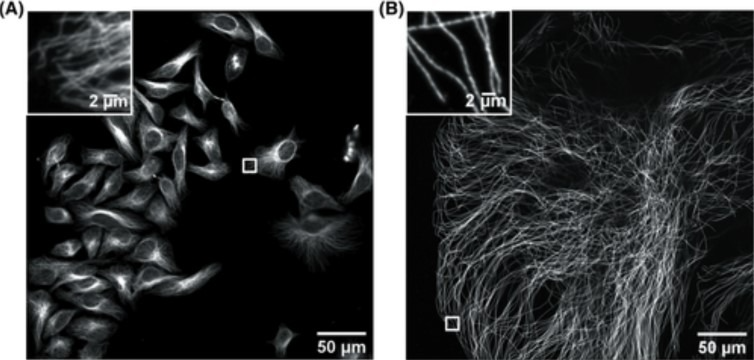
Figure 1. Confocal image of HeLa cells non expanded microtubules (right) and 4.5x linearly expanded microtubules (left). Imaging was performed on an Andor spinning-disk confocal microscope (Dragonfly) with a 40×, numerical aperture (NA) 1.15 water- immersion objective. (A) Confocal image of HeLa cells with immunostained microtubules imaged at a single XY plane at the bottom of the cells. The inset in the upper left zooms in on the small box at the middle right. (B) Confocal image of a ∼4.5× linearly expanded HeLa cell with immunostained microtubules imaged at a single XY plane at the bottom of the cell. The inset in the upper left zooms in on the small box at the bottom left. Scale bars in (B) indicate post-expansion scales. Only a fraction of an expanded cell fills the entire field of view. The respective insets display a zoom of the respective small boxes of the full field of view. (Zhang, C., et al, Current Protocols in Neuroscience, 2020). Image Credit: Andor Technology Ltd.
ExM is highly cost-effective, and this sample preparation method involves synthesizing a dense interconnected web of swellable polymer within the biological specimen of interest. The tissue inside the polymer matrix can be expanded and labeled and then when immersed in water, this expansion isotropically pulls apart the cellular structures, resulting in large gaps between each biomolecule.
The specimen is magnified isotropically, meaning that an effective higher resolution can be achieved with a standard microscope. A 4x linear expansion is reported in pure water, meaning a 64x volumetric expansion. Employing an Andor spinning disk confocal (Dragonfly) on an expanded microtubule Hela cells sample (Figure 1) illustrates how the use of expansion microscopy protocols can reveal previously unseen features in samples.
Extra care should be taken when preparing expanded samples, however, and successfully implementing expansion microscopy protocols relies on a number of considerations:
Embedding safely - a non-invasive methodology must be used to insert the polymer into cells. This is achieved by carefully inserting small monomers into the cells and tissues - these monomers are building blocks of the hydrogel that will be expanded, so the monomer polymerization must only be triggered once these are fully inside the preserved cells and tissues.
Expanding without de-structuring – the sample must be expanded whilst maintaining the structural organization of cells. To ensure that the sample is not distorted during expansion, the specimen is treated with heat/detergent or enzymatic digestion in order to mechanically homogenize the specimen, ensuring that the organization remains intact.
Table 2. Comparison of Expansion microscopy protocols. See the glossary above for the abbreviations.
| Protocol name |
ExM
(Boyden Lab) |
ExM (Vaughan Lab) |
ProExM
(Boyden
Lab) |
MAP
(Chung
Lab) |
ExFiSH (Boyden Lab) |
| Hydrogel |
acrylamide + sodium acrylate +++ MBAA |
acrylamide + sodium acrylate +++ |
acrylamide + sodium acrylate +++ |
acrylamide +++ sodium acrylate + BA |
acrylamide sodium acrylate MBAA |
Linking
agent |
acrydite |
MA-NHS GA |
acryloyle-X (AcX) |
paraformaldehyde acrylamide |
LabelX (AcX + Label-IT amine) |
| Disruption agent |
proteinase K |
proteinase K |
proteinase K |
SDS |
proteinase K |
| Disruption type |
digestion |
digestion |
digestion or gentle disruption |
denaturation dissociation |
digestion |
| Expansion factor (linear) |
4.5 |
4.0-4.2 |
4 |
4 |
3.3 |
| Resolution |
70 nm |
65 nm |
70 nm |
60 nm |
/ |
| FP preservation |
no |
yes |
yes (50% intensity) |
no |
no |
| IF staining |
no |
yes |
yes |
yes |
no |
| Sample |
cells, brain tissue |
cells, brain tissue |
cells, brain, pancreas, lung, spleen tissues |
cells, brain, spinal cord, lung, heart, liver, kidney, intestine tissues |
cells, brain tissue |
| Target |
proteins |
proteins & DNA |
proteins |
proteins & saccharides |
RNA & DNA |
| Pros comments |
the first method reported |
conventional fluorophores organelle level |
conventional fluorophores post-expansion labeling |
post-expansion labeling whole organ level multiplexed staining |
3D imaging post-expansion FISH multiplexing & HCR amplification |
| Cons comments |
complex protocol no standard fluorophores |
pre-expansion labeling only fluorescence loss |
incomplete homogenization fluorescence loss |
incompatibility
w/FPs
preparation
lost after
3 days |
/ |
| Reference |
Chen et al., 2015 Science |
Chozonski et al. 2016 Nature Methods |
Tillberg et al., 2016 Nature Biotech |
Ku et al., 2016 Nature Biotch |
Chen et al., 2016 Nature Methods |
| Variant |
iExM |
x10 |
x10; DiExM |
uExM |
|
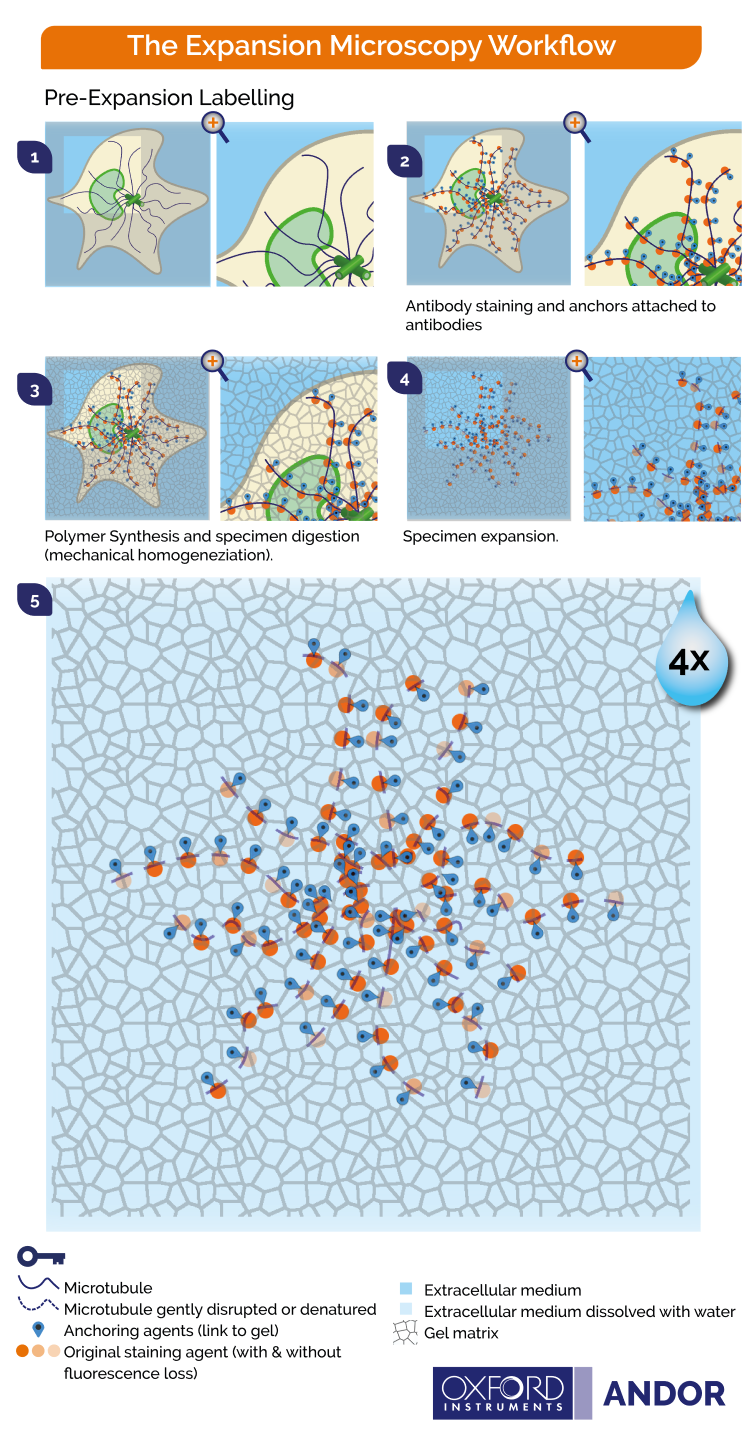
Figure 2. Principles of pre-expansion microscopy. 1) Cells are fixed 2) Labelling by immunostaining is performed on the samples and biomolecules are covalently anchored to the gel 3) During gelation the specimen is immersed in a monomer solution and the chemical network is formed. 4) During homogenization, the specimen structures are chopped by enzymatic digestion to ensure that the organization is kept intact. 5) Upon water immersion, spontaneous expansion occurs. A 4-fold linear expansion is reported in pure water (64 volumetric expansion). Image Credit: Andor Technology Ltd.
There are two primary approaches to expansion microscopy:
- Staining the biomolecules before expanding the tissue (Figure 2)
- Expanding the tissue before staining the biomolecules (Figure 3)
Figures 2 and 3 display protocols in which cells and tissues can be expanded up to 4x. The 200 nm diffraction limit of light dictates what can be distinguished as separated by light microscopes, and the linear expansion achieved is a factor of 4. Biomolecules that are separated up to 50 nm will be visible under the light microscope when using these protocols.
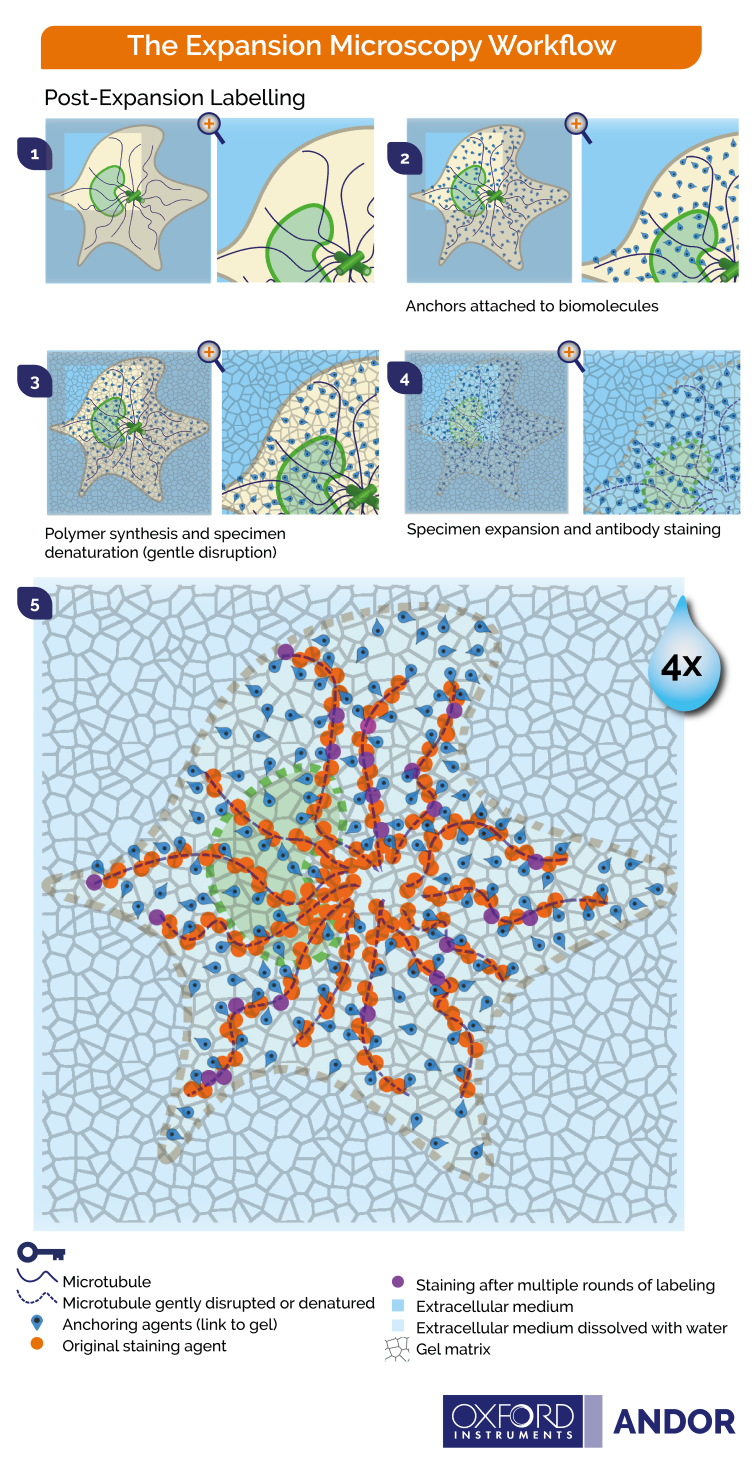
Figure 3. Principles of post-expansion microscopy 1) Fixation of cells 2) Covalent anchoring of endogenous proteins to the gel 3) Hydrogel embedding with high polyacrylamide concentration 4) Denaturation and dissociation of non-crosslinked proteins 5) Expansion upon water immersion 6) Post-expansion immunostaining possible in multiple rounds. Image Credit: Andor Technology Ltd.
Fundamental Steps of Expansion Microscopy
The fluorescent signal is also isotropically expanded through expansion, leading to an effective higher resolution. Key steps for expansion microscopy protocols are outlined below and in Figures 2 and 3.
- Labeling - fluorophores are used to tag biomolecules, though in post-expansion labeling this step would be performed at the end, after expansion.
- Anchoring - biomolecules and/or labels are covalently fitted with molecular handles, and these cross-linkers will enable the polymer matrix to exercise force on the biomolecules.
- Gelation - here, the polymer chain is broken down into its constituent parts, or building blocks, to avoid damage to the cells. The specimen is then immersed in a monomer solution (sodium acrylate) and a penetrating hydrogel (sodium polyacrylate). A chemical reaction is triggered once the solution and hydrogen are inside the cells, causing the monomers to bind and form a network of the desired polymer.
- Homogenization – this step aims to avert sample distortion and ensure that specimen organization is maintained. The sample will be chemically disrupted by enzymatic digestion or via a heat and detergent treatment (denaturation/dissociation). The homogenization treatment used will depend on the nature of the specimen and the specific molecules to be visualized.
- Expansion - the specimen is immersed in water, and the water diffuses into the polyelectrolyte hydrogel via osmotic force, promoting polymer expansion. The expansion will pull apart anchored biomolecules in an isotropic fashion, creating substantial gaps between individual biomolecules. The expanded specimen’s spatial organization is preserved, therefore allowing nanoscale imaging using standard fluorescent microscopes.
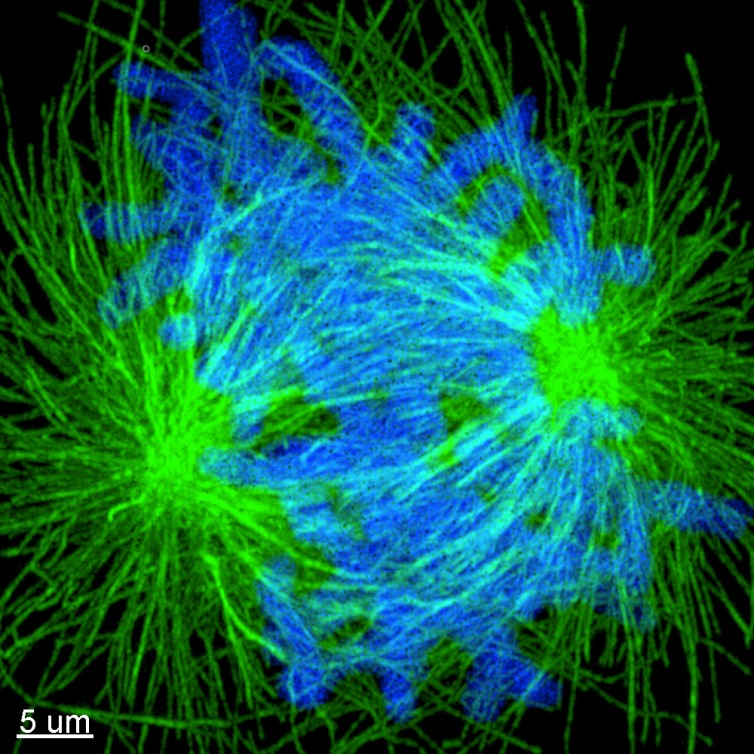
Figure 4. Image of expanded mitotic cell. Maximum projection images of dividing cells stained for tubulin (green) and DNA (Blue). Cells where labelled using the post-expansion protocol, and image with Andor Dragonfly. Image Credit: Sample courtesy of Joshua C Vaughan (University of Washington).
Most Popular Protocols of Expansion Microscopy
Numerous different Expansion Microscopy protocols have been published since its initial development, and there are currently variations and improvements encompassing a wide range of applications. The table below summarizes the key protocols for expansion microscopy.
To summarize; two main strategies of expansion microscopy exist side by side: pre and post-expansion labeling strategies (Figures 2 and 3). These different protocols are adapted to certain cellular structures, so when beginning to use expansion microscopy imaging, appropriate care must be taken to select the most appropriate protocol for visualizing the subcellular structure in question.
It may be necessary to optimize a protocol in order to better suit experimental conditions, and researchers are advised to design proper experimental controls in order to demonstrate the isotropic expansion of the structure to be analyzed.
References and Further Reading
- Chang, J.-B., et al. (2017). " Iterative expansion microscopy." Nature Methods 14(6): 593-599.
- Chen, F., et al. (2015). "Expansion microscopy." Science 347(6221): 543-548.
- Chen, F., et al. (2016). "Nanoscale imaging of RNA with expansion microscopy." Nature Methods 13(8): 679-684
- Chozinski, T. J., et al. (2016). "Expansion microscopy with conventional antibodies and fluorescent proteins." Nature Methods 13(6): 485-488
- Gambarotto, D., et al. (2019). "Imaging cellular ultrastructures using expansion microscopy (U-ExM)." Nature Methods 16(1): 71-74
- Ku T, Swaney J, Park JY, et al. (2016 ) “Multiplexed and scalable super-resolution imaging of three-dimensional protein localization in size-adjustable tissues.” Nat Biotechnol. 34 (9):973-981. doi:10.1038/nbt.3641
- Schermelleh, L., et al. (2019). "Super-resolution microscopy demystified." Nature Cell Biology 21(1): 72-84.
- Schermelleh, L., et al. (2010). "A guide to super-resolution fluorescence microscopy." Journal of Cell Biology 190(2): 165-175. Pernal, S. P., et al. (2019). "Differential expansion microscopy." bioRxiv: 699579.
- Truckenbrodt, S., et al. (2018). "X10 expansion microscopy enables 25-nm resolution on conventional microscopes." EMBO reports 19(9): e45836.
- Truckenbrodt, S., et al. (2019). "A practical guide to optimization in X10 expansion microscopy." Nature Protocols 14(3): 832-863.
- Tillberg PW, Chen F, Piatkevich KD, et al. (2016) " Protein-retention expansion microscopy of cells and tissues labeled using standard fluorescent proteins and antibodies. " Nat Biotechnol. 34 (9):987-992.
- Wassie, A. T., et al. (2019). "Expansion microscopy: principles and uses in biological research." Nature Methods 16(1): 33-41
- Zhang, C., Kang, J. S., Asano, S. M., Gao, R., & Boyden, E. S. (2020) “Expansion microscopy for beginners: Visualizing microtubules in expanded cultured HeLa cells.” Current Protocols in Neuroscience
Acknowledgments
Produced from materials originally authored by Dr. Sébastien Bellow and Dr. Claudia Florindo from Oxford Instruments Andor.

This information has been sourced, reviewed and adapted from materials provided by Andor Technology Ltd.
For more information on this source, please visit Andor Technology Ltd.